Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis
- PMID: 9050882
- PMCID: PMC20020
- DOI: 10.1073/pnas.94.5.1931
Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis
Abstract
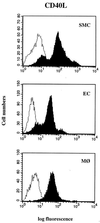
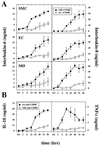
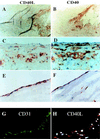
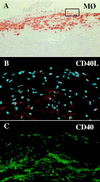
Increasing evidence supports involvement of inflammation and immunity in atherogenesis. We report here that CD40 ligand (CD40L), an immunoregulatory signaling molecule heretofore considered largely restricted to recently activated CD4+ T lymphocytes, is expressed by human vascular endothelial cells (EC), smooth muscle cells (SMC), and human macrophages in vitro, and is coexpressed with its receptor CD40 on all three cells types in human atherosclerotic lesions in situ. Cultured human vascular EC, SMC, and human macrophages all constitutively expressed CD40L mRNA as well as protein. Stimulation with interleukin 1beta, tumor necrosis factor alpha, or interferon gamma increased surface levels and de novo synthesis of CD40L on all three cell types. CD40L expressed on EC, SMC, and macrophages exhibited biological activity, as it induced B7.2 expression on B cells. Human vascular SMC also constitutively expressed CD40, the receptor for CD40L, and through CD40 signaling, human recombinant CD40L induced expression of proinflammatory cytokines in these cells, identifying SMC as a target for CD40L. Human atherosclerotic lesions (n = 8) showed expression of immunoreactive CD40L on EC, SMC, and macrophages, while normal arterial tissues (n = 5) contained no CD40L. In atheroma CD40L+ cells often also expressed CD40. These observations establish human vascular EC, SMC, and human macrophages as a novel source of CD40L, and point to T cell-independent CD40 signaling, and a broader function of this pathway in regulation of nonimmune cells, as illustrated here by potential autocrine and paracrine activation during atherogenesis.
Figures

Similar articles
-
Expression of stromelysin-3 in atherosclerotic lesions: regulation via CD40-CD40 ligand signaling in vitro and in vivo.J Exp Med. 1999 Mar 1;189(5):843-53. doi: 10.1084/jem.189.5.843. J Exp Med. 1999. PMID: 10049948 Free PMC article.
-
CD40 ligation induces tissue factor expression in human vascular smooth muscle cells.Am J Pathol. 2000 Jan;156(1):7-14. doi: 10.1016/S0002-9440(10)64699-8. Am J Pathol. 2000. PMID: 10623647 Free PMC article.
-
CD40 and CD40 ligand (CD154) are coexpressed on microvessels in vivo in human cardiac allograft rejection.Transplantation. 1997 Dec 27;64(12):1765-74. doi: 10.1097/00007890-199712270-00025. Transplantation. 1997. PMID: 9422418
-
CD40 signaling in vascular cells: a key role in atherosclerosis?Atherosclerosis. 1998 Apr;137 Suppl:S89-95. doi: 10.1016/s0021-9150(97)00309-2. Atherosclerosis. 1998. PMID: 9694547 Review.
-
CD40 signaling and plaque instability.Circ Res. 2001 Dec 7;89(12):1092-103. doi: 10.1161/hh2401.101272. Circ Res. 2001. PMID: 11739273 Review.
Cited by
-
The role of neurotrophins in multiple sclerosis-pathological and clinical implications.Int J Mol Sci. 2012 Oct 22;13(10):13713-25. doi: 10.3390/ijms131013713. Int J Mol Sci. 2012. PMID: 23202976 Free PMC article. Review.
-
Elevated circulating inflammatory biomarker levels in the SIRT1-NF-κB-sCD40L pathway in patients with acute myocardial infarction: a case-control study.Ann Med. 2023;55(2):2284366. doi: 10.1080/07853890.2023.2284366. Epub 2023 Nov 22. Ann Med. 2023. PMID: 37992411 Free PMC article.
-
Glucocorticoid-induced tumour necrosis factor receptor family related protein (GITR) mediates inflammatory activation of macrophages that can destabilize atherosclerotic plaques.Immunology. 2006 Nov;119(3):421-9. doi: 10.1111/j.1365-2567.2006.02453.x. Immunology. 2006. PMID: 17067317 Free PMC article.
-
T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm.Am J Pathol. 2002 Aug;161(2):499-506. doi: 10.1016/S0002-9440(10)64206-X. Am J Pathol. 2002. PMID: 12163375 Free PMC article.
-
Advances in immunotherapy modalities for atherosclerosis.Front Pharmacol. 2023 Jan 10;13:1079185. doi: 10.3389/fphar.2022.1079185. eCollection 2022. Front Pharmacol. 2023. PMID: 36703734 Free PMC article. Review.
References
-
- Armitage R J, Fanslow W C, Strockbine L, Sato T A, Clifford K N, Macduff B M, Anderson D M, Gimpel S D, Davis-Smith T, Maliszewski C R, Clark E A, Smith C A, Grabstein K H, Cosman D, Spriggs M K. Nature (London) 1992;357:80–82. - PubMed
-
- Graf D, Korthauer U, Mages H W, Senger G, Kroczek R A. Eur J Immunol. 1992;22:3191–3194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

