Development of "substrate-trapping" mutants to identify physiological substrates of protein tyrosine phosphatases
- PMID: 9050838
- PMCID: PMC19976
- DOI: 10.1073/pnas.94.5.1680
Development of "substrate-trapping" mutants to identify physiological substrates of protein tyrosine phosphatases
Abstract
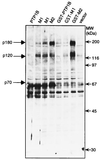
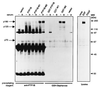
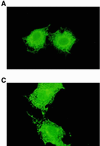
The identification of substrates of protein tyrosine phosphatases (PTPs) is an essential step toward a complete understanding of the physiological function of members of this enzyme family. PTPs are defined by a conserved catalytic domain harboring 27 invariant residues. From a mutagenesis study of these invariant residues that was guided by our knowledge of the crystal structure of PTP1B, we have discovered a mutation of the invariant catalytic acid (Asp-181 in PTP1B) that converts an extremely active enzyme into a "substrate trap." Expression of this D181A mutant of PTP1B in COS and 293 cells results in an enzyme that competes with endogenous PTP1B for substrates and promotes the accumulation of phosphotyrosine primarily on the epidermal growth factor (EGF) receptor as well as on proteins of 120, 80, and 70 kDa. The association between the D181A mutant of PTP1B and these substrates was sufficiently stable to allow isolation of the complex by immunoprecipitation. As predicted for an interaction between the substrate-binding site of PTP1B and its substrates, the complex is disrupted by vanadate and, for the EGF receptor, the interaction absolutely requires receptor autophosphorylation. Furthermore, from immunofluorescence studies, the D181A mutant of PTP1B appeared to retain the endogenous EGF receptor in an intracellular complex. These results suggest that the EGF receptor is a bona fide substrate for PTP1B in vivo and that one important function of PTP1B is to prevent the inappropriate, ligand-independent, activation of newly synthesized EGF receptor in the endoplasmic reticulum. This essential catalytic aspartate residue is present in all PTPs and has structurally equivalent counterparts in the dual-specificity phosphatases and the low molecular weight PTPs. Therefore we anticipate that this method may be widely applicable to facilitate the identification of substrates of other members of this enzyme family.
Figures




Similar articles
-
Design and characterization of an improved protein tyrosine phosphatase substrate-trapping mutant.Biochemistry. 2002 Mar 26;41(12):4032-9. doi: 10.1021/bi015904r. Biochemistry. 2002. PMID: 11900546
-
Epidermal growth factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase.Mol Cell Biol. 1998 Mar;18(3):1622-34. doi: 10.1128/MCB.18.3.1622. Mol Cell Biol. 1998. PMID: 9488479 Free PMC article.
-
Protein tyrosine phosphatase 1B interacts with and is tyrosine phosphorylated by the epidermal growth factor receptor.Biochem J. 1997 Oct 1;327 ( Pt 1)(Pt 1):139-45. doi: 10.1042/bj3270139. Biochem J. 1997. PMID: 9355745 Free PMC article.
-
Protein tyrosine phosphatase function: the substrate perspective.Biochem J. 2007 Feb 15;402(1):1-15. doi: 10.1042/BJ20061548. Biochem J. 2007. PMID: 17238862 Free PMC article. Review.
-
The Extended Family of Protein Tyrosine Phosphatases.Methods Mol Biol. 2016;1447:1-23. doi: 10.1007/978-1-4939-3746-2_1. Methods Mol Biol. 2016. PMID: 27514797 Review.
Cited by
-
Redox regulation of protein kinases.Crit Rev Biochem Mol Biol. 2013 Jul-Aug;48(4):332-56. doi: 10.3109/10409238.2013.790873. Epub 2013 May 3. Crit Rev Biochem Mol Biol. 2013. PMID: 23639002 Free PMC article. Review.
-
Protein tyrosine phosphatase 1B (PTP1B) modulates palmitate-induced cytokine production in macrophage cells.Inflamm Res. 2013 Feb;62(2):239-46. doi: 10.1007/s00011-012-0573-0. Epub 2012 Nov 15. Inflamm Res. 2013. PMID: 23229720
-
THEMIS is a substrate and allosteric activator of SHP1, playing dual roles during T cell development.Nat Struct Mol Biol. 2024 Jan;31(1):54-67. doi: 10.1038/s41594-023-01131-3. Epub 2024 Jan 4. Nat Struct Mol Biol. 2024. PMID: 38177672
-
Voltage sensitive phosphatases: emerging kinship to protein tyrosine phosphatases from structure-function research.Front Pharmacol. 2015 Jan 10;6:20. doi: 10.3389/fphar.2015.00020. eCollection 2015. Front Pharmacol. 2015. PMID: 25713537 Free PMC article. Review.
-
Regulation of insulin receptor signaling by the protein tyrosine phosphatase TCPTP.Mol Cell Biol. 2003 Mar;23(6):2096-108. doi: 10.1128/MCB.23.6.2096-2108.2003. Mol Cell Biol. 2003. PMID: 12612081 Free PMC article.
References
-
- Tonks N K, Neel B G. Cell. 1996;87:365–368. - PubMed
-
- Tonks N K. Adv Pharmacol. 1996;36:91–119. - PubMed
-
- Barford D, Jia Z, Tonks N K. Nat Struct Biol. 1995;2:1043–1053. - PubMed
-
- Tonks N K, Diltz C D, Fischer E H. J Biol Chem. 1988;263:6722–6730. - PubMed
-
- Tonks N K, Diltz C D, Fischer E H. J Biol Chem. 1988;263:6731–6737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

