Cross-talk between thyroid hormone and specific retinoid X receptor subtypes in yeast selectively regulates cognate ligand actions
- PMID: 9041123
- PMCID: PMC6148309
Cross-talk between thyroid hormone and specific retinoid X receptor subtypes in yeast selectively regulates cognate ligand actions
Abstract
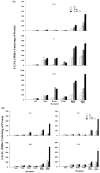
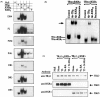
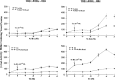
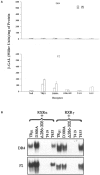
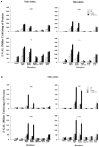
Thyroid (T3) hormone beta1 (TR) and 9-cis retinoic acid (9c-RA) retinoid X receptors (RXR) can form heterodimer complexes that bind to hormone response elements (HREs) in target genes to either activate or repress transcription. However, the action of each cognate ligand and the accessory cellular factors that can differentially regulate the transcriptional responses of a heterodimer-DNA complex are not well understood. Studies in most mammalian cell lines have demonstrated that 9c-RA cannot bind or transactivate TR/RXR-T3 response element (TRE) complexes. In contrast, when identical heterodimer complexes were coexpressed in the yeast (Saccharomyces cerevisiae) with single copy typical TREs [i.e., DR+4 (direct repeat), F2 (everted repeat), or PAL (inverted repeat) DNA response elements] we observed that i) unliganded TRbeta1 homodimers had constitutive action on F2 and PAL but not DR4 TREs; ii) TRbeta1 homodimer responsivity to T3 ligand was relatively weak (less than twofold) and was only demonstrable on F2 but not PAL or DR4-TREs, whereas TRbeta1 heterodimers responded to T3 when RXRgamma but not RXR alpha was the heterodimeric partner; iii) RXR responsivity to 9c-RA (three- to sixfold) could be demonstrated only on palindromic TREs that could be enhanced by TRbeta1 on all TREs; iv) T3 + 9c-RA ligands increased (additively or synergistically) transactivation when RXRgamma but not alpha heterodimerized with TRbeta1 on both typical as well as atypical (DR1, DR3, DR5, and F2M) TREs. Substitutions for wild-type TRbeta1 of C-terminus mutants deficient in dimerization with RXRs abrogated the anticipated single and dual cognate ligand-induced effects on TRbeta1/RXRgamma transactivation of DR4 TREs, whereas mutants with preserved dimerization function but impaired T3 transactivation regions could maintain an enhanced 9c-RA response but were devoid of the anticipated T3 and dual (T3 + 9c-RA) cognate ligand-induced effects. Thus, the ligand-inducible response of TR and RXR homodimers expressed in yeast are relatively weak but can be further enhanced by TRbeta1 cross-talk with specific RXR subtypes in the presence of both cognate ligands.
Figures

Similar articles
-
The function of retinoid X receptors on negative thyroid hormone response elements.Mol Cell Endocrinol. 1997 Apr 4;128(1-2):85-96. doi: 10.1016/s0303-7207(97)04025-2. Mol Cell Endocrinol. 1997. PMID: 9140079
-
Roles of 3,5,3'-triiodothyronine and deoxyribonucleic acid binding on thyroid hormone receptor complex formation.Endocrinology. 1994 Mar;134(3):1075-81. doi: 10.1210/endo.134.3.8119145. Endocrinology. 1994. PMID: 8119145
-
Ligand- and DNA-induced dissociation of RXR tetramers.J Mol Biol. 1998 Jan 9;275(1):55-65. doi: 10.1006/jmbi.1997.1413. J Mol Biol. 1998. PMID: 9451439
-
Thyroid hormone and thyroid hormone nuclear receptors: History and present state of art.Endocr Regul. 2021 May 21;55(2):103-119. doi: 10.2478/enr-2021-0012. Endocr Regul. 2021. PMID: 34020531 Review.
-
How do thyroid hormone receptors bind to structurally diverse response elements?Mol Cell Endocrinol. 1994 Apr;100(1-2):125-31. doi: 10.1016/0303-7207(94)90291-7. Mol Cell Endocrinol. 1994. PMID: 8056146 Review.
Cited by
-
Human nuclear receptor heterodimers: opportunities for detecting targets of transcriptional regulation using yeast.Gene Expr. 1996;5(4-5):255-68. Gene Expr. 1996. PMID: 8723391 Free PMC article. Review.
-
Yeast hormone response element assays detect and characterize GRIP1 coactivator-dependent activation of transcription by thyroid and retinoid nuclear receptors.Proc Natl Acad Sci U S A. 1997 Apr 15;94(8):3697-702. doi: 10.1073/pnas.94.8.3697. Proc Natl Acad Sci U S A. 1997. PMID: 9108040 Free PMC article.
References
-
- Allegretto E. A.; McClurg M. R.; Lazarchik S. B.; Clemm D. L.; Kerner S. A.; Elgort M. G.; Boehm M. F .; White S. K.; Pike J. W.; Heyman R.A. Transactivation properties of retmoic acid and retinoid X receptors in mammalian cells and yeast: Correlation with hormone binding and effects of metabolism. J. Biol. Chem. 268:26625–26633; 1993. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
