Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes
- PMID: 9016874
- PMCID: PMC2196124
- DOI: 10.1084/jem.185.2.251
Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes
Abstract
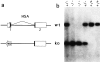
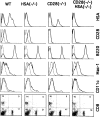
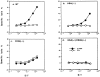
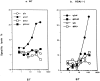
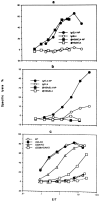
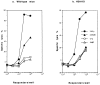
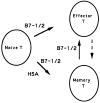
A successful T cell immune response has two major products: effector T cells which directly or indirectly remove the antigens, and memory T cells, which allow a faster and more efficient recall response when challenged by related antigens. An important issue is whether costimulatory molecules on the antigen-presenting cells are involved in determining whether T cells will differentiate into effector or memory cells after antigenic stimulation. To address this issue, we have produced mice with targeted mutations of either the heat-stable antigen (HSA), or both HSA and CD28. We show that CD28/B7 and HSA provide two alternative costimulatory pathways for induction of immunological memory to influenza virus. Furthermore, our results revealed that B7 is essential for the generation of effector T cells from either naive or memory T cells, while HSA is not necessary for the generation of effector T cells. Our results demonstrate that the induction of memory T cells and effector T cells can utilize distinct costimulatory molecules. These results have important implications on lineage relationship between effector and memory T cells.
Figures


Similar articles
-
T cell costimulation by B7/BB1 induces CD8 T cell-dependent tumor rejection: an important role of B7/BB1 in the induction, recruitment, and effector function of antitumor T cells.J Exp Med. 1994 Apr 1;179(4):1205-14. doi: 10.1084/jem.179.4.1205. J Exp Med. 1994. PMID: 7511683 Free PMC article.
-
CD28-B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help.J Exp Med. 1993 Jun 1;177(6):1791-6. doi: 10.1084/jem.177.6.1791. J Exp Med. 1993. PMID: 7684435 Free PMC article.
-
Memory alloreactive cytotoxic T cells do not require costimulation for activation in vitro.Immunol Cell Biol. 1996 Oct;74(5):413-20. doi: 10.1038/icb.1996.71. Immunol Cell Biol. 1996. PMID: 8912004
-
Aspects of cytotoxic T cell memory.Immunol Rev. 1996 Apr;150:113-27. doi: 10.1111/j.1600-065x.1996.tb00698.x. Immunol Rev. 1996. PMID: 8782704 Review.
-
T Cell Activation Pathways: B7, LFA-3, and ICAM-1 Shape Unique T Cell Profiles.Crit Rev Immunol. 2017;37(2-6):463-481. doi: 10.1615/CritRevImmunol.v37.i2-6.130. Crit Rev Immunol. 2017. PMID: 29773030 Review.
Cited by
-
Antigen processing and CD24 expression determine antigen presentation by splenic CD4+ and CD8+ dendritic cells.Immunology. 2008 Mar;123(3):447-55. doi: 10.1111/j.1365-2567.2007.02711.x. Epub 2007 Oct 19. Immunology. 2008. PMID: 17949418 Free PMC article.
-
ThPOK derepression is required for robust CD8 T cell responses to viral infection.J Immunol. 2009 Oct 1;183(7):4467-74. doi: 10.4049/jimmunol.0901428. Epub 2009 Sep 4. J Immunol. 2009. PMID: 19734230 Free PMC article.
-
CD40 ligand-mediated interactions are involved in the generation of memory CD8(+) cytotoxic T lymphocytes (CTL) but are not required for the maintenance of CTL memory following virus infection.J Virol. 1998 Sep;72(9):7440-9. doi: 10.1128/JVI.72.9.7440-7449.1998. J Virol. 1998. PMID: 9696840 Free PMC article.
-
Tyrosine-phosphorylation-dependent translocation of the SLAT protein to the immunological synapse is required for NFAT transcription factor activation.Immunity. 2008 Nov 14;29(5):704-19. doi: 10.1016/j.immuni.2008.08.015. Epub 2008 Oct 30. Immunity. 2008. PMID: 18976935 Free PMC article.
-
CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host.J Exp Med. 2004 Oct 18;200(8):1083-9. doi: 10.1084/jem.20040779. Epub 2004 Oct 11. J Exp Med. 2004. PMID: 15477346 Free PMC article.
References
-
- Ada GL, Jones PD. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. - PubMed
-
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus-persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature (Lond) 1993;362:758–761. - PubMed
-
- Tripp RA, Lahti JM, Doherty PC. Laser light suicide of proliferating virus-specific CD8 T cells in an in vivoresponse. J Immunol. 1995;155:3719–3721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

