Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles
- PMID: 8918538
- PMCID: PMC6953178
- DOI: 10.1006/viro.1996.0579
Identification of the human cytomegalovirus G protein-coupled receptor homologue encoded by UL33 in infected cells and enveloped virus particles
Abstract
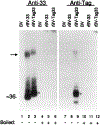
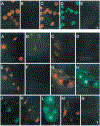
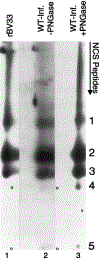
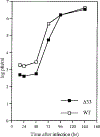
Human cytomegalovirus (HCMV), strain AD169, contains four genes (US27, US28, UL33, and UL78) that encode putative homologues of cellular G protein-coupled receptors (GCRs). GCRs transduce extracellular signals to alter intracellular processes, and there is evidence that HCMV may elicit such changes at early times following infection. The US27, US28, and UL33 genes are transcribed during infection, and the US28 gene product has been found to be a functional receptor for the beta-chemokine class of immune modulators. The US27, UL33, and UL78 gene products have not been described and we have concentrated on identifying the UL33 protein because it is the most highly conserved of the GCR homologues among the human beta and gamma herpesviruses. We report here cloning UL33 into a recombinant baculovirus (rBV) and expressing it in insect cells; constructing a mutant HCMV with a disrupted UL33 gene; and identifying the UL33 protein in HCMV-infected cells and virus particles. Our results demonstrate that the UL33 protein (i) is expressed as a approximately 36-kDa, heat-aggregatable protein in rBV-infected cells, (ii) is modified heterogeneously by asparagine-linked glycosylation and expressed as a > or = 58-kDa glycoprotein that is present in the region of the cytoplasmic inclusions in HCMV-infected fibroblasts, (iii) is present in virions and two other enveloped virus particles, and (iv) is not essential for growth of HCMV in human foreskin fibroblast cultures.
Figures



Similar articles
-
The chemokine receptor homologue encoded by US27 of human cytomegalovirus is heavily glycosylated and is present in infected human foreskin fibroblasts and enveloped virus particles.Virus Res. 2007 Jan;123(1):57-71. doi: 10.1016/j.virusres.2006.08.003. Epub 2006 Sep 8. Virus Res. 2007. PMID: 16963142 Free PMC article.
-
The Human Cytomegalovirus US27 Gene Product Constitutively Activates Antioxidant Response Element-Mediated Transcription through Gβγ, Phosphoinositide 3-Kinase, and Nuclear Respiratory Factor 1.J Virol. 2018 Nov 12;92(23):e00644-18. doi: 10.1128/JVI.00644-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209167 Free PMC article.
-
Human Cytomegalovirus-Encoded G Protein-Coupled Receptor UL33 Facilitates Virus Dissemination via the Extracellular and Cell-to-Cell Route.Viruses. 2020 May 30;12(6):594. doi: 10.3390/v12060594. Viruses. 2020. PMID: 32486172 Free PMC article.
-
Emerging roles of cytomegalovirus-encoded G protein-coupled receptors during lytic and latent infection.Med Microbiol Immunol. 2019 Aug;208(3-4):447-456. doi: 10.1007/s00430-019-00595-9. Epub 2019 Mar 21. Med Microbiol Immunol. 2019. PMID: 30900091 Review.
-
HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks.Trends Pharmacol Sci. 2006 Jan;27(1):56-63. doi: 10.1016/j.tips.2005.11.006. Epub 2005 Dec 13. Trends Pharmacol Sci. 2006. PMID: 16352349 Review.
Cited by
-
Receptor chimeras demonstrate that the C-terminal domain of the human cytomegalovirus US27 gene product is necessary and sufficient for intracellular receptor localization.Virol J. 2012 Feb 16;9:42. doi: 10.1186/1743-422X-9-42. Virol J. 2012. PMID: 22339884 Free PMC article.
-
Activation of herpesvirus gene expression by the human cytomegalovirus protein pp71.J Virol. 1999 Oct;73(10):8512-8. doi: 10.1128/JVI.73.10.8512-8518.1999. J Virol. 1999. PMID: 10482604 Free PMC article.
-
Herpesvirus homologues of cellular genes.Virus Genes. 2000;21(1-2):65-75. Virus Genes. 2000. PMID: 11022790 Review.
-
Desensitization of herpesvirus-encoded G protein-coupled receptors.Life Sci. 2008 Jan 16;82(3-4):125-34. doi: 10.1016/j.lfs.2007.10.024. Epub 2007 Nov 13. Life Sci. 2008. PMID: 18054964 Free PMC article. Review.
-
Murine cytomegalovirus M78 protein, a G protein-coupled receptor homologue, is a constituent of the virion and facilitates accumulation of immediate-early viral mRNA.Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3237-42. doi: 10.1073/pnas.051629898. Proc Natl Acad Sci U S A. 2001. PMID: 11248062 Free PMC article.
References
-
- Ahuja SK, Gao J-L, and Murphy PM (1994). Chemokine receptors and molecular mimicry. Immunol. Today 15, 281–287. - PubMed
-
- Ahuja SK, and Murphy PM (1993). Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J. Biol. Chem 268, 20691–20694. - PubMed
-
- Albrecht T, Boldogh I, Fons MP, Deng CZ, AbuBakar S, and Millinoff D (1991). Role of immediate early cellular responses in the initiation of cytomegalovirus infection In “Progress in Cytomegalovirus Research” (Landini MP, Ed.), pp. 239–257. Excerpta Medica, Amsterdam.
-
- Alford CA, and Britt WJ (1990). Cytomegalovirus In “Virology” (Fields BN and Knipe DM, Eds.), 2nd ed., pp. 1981–2010. Raven Press, New York.
-
- Anker HS (1970). A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 7, 293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

