Coordinated expression of dopamine receptors in neostriatal medium spiny neurons
- PMID: 8815934
- PMCID: PMC6578920
- DOI: 10.1523/JNEUROSCI.16-20-06579.1996
Coordinated expression of dopamine receptors in neostriatal medium spiny neurons
Abstract
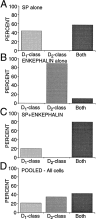
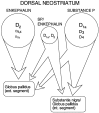
In recent years, the distribution of dopamine receptor subtypes among the principal neurons of the neostriatum has been the subject of debate. Conventional anatomical and physiological approaches have yielded starkly different estimates of the extent to which D1 and D2 class dopamine receptors are colocalized. One plausible explanation for the discrepancy is that some dopamine receptors are present in physiologically significant numbers, but the mRNA for these receptors is not detectable with conventional techniques. To test this hypothesis, we examined the expression of DA receptors in individual neostriatal neurons by patch-clamp and RT-PCR techniques. Because of the strong correlation between peptide expression and projection site, medium spiny neurons were divided into three groups on the basis of expression of mRNA for enkephalin (ENK) and substance P (SP). Neurons expressing detectable levels of SP but not ENK had abundant mRNA for the D1a receptor. A subset of these cells (approximately 50%) coexpressed D3 or D4 receptor mRNA. Neurons expressing detectable levels of ENK but not SP had abundant mRNA for D2 receptor isoforms (short and long). A subset (10-25%) of these neurons coexpressed D1a or D1b mRNAs. Neurons coexpressing ENK and SP mRNAs consistently coexpressed D1a and D2 mRNAs in relatively high abundance. Functional analysis of neurons expressing lower abundance mRNAs revealed clear physiological consequences that could be attributed to these receptors. These results suggest that, although colocalization of D1a and D2 receptors is limited, functional D1 and D2 class receptors are colocalized in nearly one-half of all medium spiny projection neurons.
Figures





Similar articles
-
Coordinated expression of dopamine receptors in neostriatal medium spiny neurons.Adv Pharmacol. 1998;42:1020-3. doi: 10.1016/s1054-3589(08)60921-7. Adv Pharmacol. 1998. PMID: 9328072 No abstract available.
-
Histamine Excites Striatal Dopamine D1 and D2 Receptor-Expressing Neurons via Postsynaptic H1 and H2 Receptors.Mol Neurobiol. 2018 Oct;55(10):8059-8070. doi: 10.1007/s12035-018-0976-1. Epub 2018 Mar 1. Mol Neurobiol. 2018. PMID: 29498008
-
D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway.J Neurophysiol. 1997 Feb;77(2):1003-15. doi: 10.1152/jn.1997.77.2.1003. J Neurophysiol. 1997. PMID: 9065864
-
D1 and D2 dopamine receptor modulation of sodium and potassium currents in rat neostriatal neurons.Prog Brain Res. 1993;99:309-24. doi: 10.1016/s0079-6123(08)61354-0. Prog Brain Res. 1993. PMID: 7906427 Review.
-
Are neostriatal dopamine receptors co-localized?Trends Neurosci. 1993 Aug;16(8):299-305. doi: 10.1016/0166-2236(93)90103-s. Trends Neurosci. 1993. PMID: 7691003 Review.
Cited by
-
Structural and functional features of medium spiny neurons in the BACHDΔN17 mouse model of Huntington's Disease.PLoS One. 2020 Jun 23;15(6):e0234394. doi: 10.1371/journal.pone.0234394. eCollection 2020. PLoS One. 2020. PMID: 32574176 Free PMC article.
-
Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats.J Neurosci. 2004 Sep 22;24(38):8289-99. doi: 10.1523/JNEUROSCI.1990-04.2004. J Neurosci. 2004. PMID: 15385612 Free PMC article.
-
Tumor suppression and improvement in immune systems by specific activation of dopamine D1-receptor-expressing neurons in the nucleus accumbens.Mol Brain. 2022 Feb 16;15(1):17. doi: 10.1186/s13041-022-00902-1. Mol Brain. 2022. PMID: 35172858 Free PMC article.
-
Different Dopaminergic Dysfunctions Underlying Parkinsonian Akinesia and Tremor.Front Neurosci. 2019 May 29;13:550. doi: 10.3389/fnins.2019.00550. eCollection 2019. Front Neurosci. 2019. PMID: 31191237 Free PMC article.
-
Phosphodiesterase 10A levels are related to striatal function in schizophrenia: a combined positron emission tomography and functional magnetic resonance imaging study.Eur Arch Psychiatry Clin Neurosci. 2020 Jun;270(4):451-459. doi: 10.1007/s00406-019-01021-0. Epub 2019 May 22. Eur Arch Psychiatry Clin Neurosci. 2020. PMID: 31119377 Free PMC article.
References
-
- Akaike A, Ohno Y, Sasa M, Takaori S. Excitatory and inhibitory effects of dopamine on neuronal activity of the caudate neurons in vitro. Brain Res. 1987;418:262–272. - PubMed
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
-
- Anderson KD, Reiner A. The patterns of neurotransmitter and neuropeptide co-occurrence among striatal projection neurons: conclusions based on recent findings. Brain Res Rev. 1990;15:251–265. - PubMed
-
- Ariano MA, Sibley DR. Dopamine receptor distribution in the rat CNS: elucidation using anti-peptide antisera directed against D1A and D3 subtypes. Brain Res. 1994;649:95–110. - PubMed
-
- Ariano MA, Larson ER, Noblett KL. Cellular dopamine receptor subtype localization. In: Ariano MA, Surmeier DJ, editors. Molecular and cellular mechanisms of neostriatal function. Landes; Austin, TX: 1995. pp. 59–70.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
