Immunohistochemical distribution and electron microscopic subcellular localization of the proteasome in the rat CNS
- PMID: 8815912
- PMCID: PMC6578903
- DOI: 10.1523/JNEUROSCI.16-20-06331.1996
Immunohistochemical distribution and electron microscopic subcellular localization of the proteasome in the rat CNS
Abstract
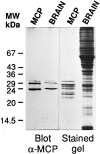
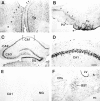
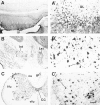
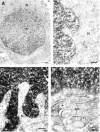
The proteasome multicatalytic proteinase (MCP) is a 20S complex that plays a major role in nonlysosomal pathways of intracellular protein degradation. A polyclonal antibody against rat liver MCP was used to investigate the distribution of MCP in the CNS of the rat and its subcellular localization within the neurons. As expected, MCP immunoreactivity (MCP-IR) was distributed ubiquitously in the rat CNS but not homogeneously. The most intensely stained neurons were the pyramidal cortical neurons of layer 5 and the motor neurons of the ventral horn in the spinal cord, which show an intense nuclear and cytoplasmatic MCP-IR and clearly stained processes. Additionally, some populations of large neurons in the mesencephalon and brainstem also displayed a moderate MCP-IR in their perikarya. The vast majority of neurons in the remaining structures did not show a strong cytoplasmatic MCP-IR, but their nuclei displayed an intense MCP-IR. The subcellular localization also was studied by immunoelectron microscopy. MCP-IR was intense in the neuronal nuclei, and significant staining also was found in the cytoplasm, dendritic, and axonic processes (including some myelinated axons) and in synaptic boutons, as illustrated in the cerebellar cortex. The distribution of MCP in the rat CNS and its subcellular localization are discussed in relation to (1) the distribution of calpain, the other major nonlysosomal cellular protease, and (2) the possible role of MCP in the degradation of regulatory proteins and key transcription factors that are essential in many neuronal responses.
Figures



Similar articles
-
Electron microscopic localization of the multicatalytic proteinase complex in rat liver and in cultured cells.J Histochem Cytochem. 1992 Aug;40(8):1165-72. doi: 10.1177/40.8.1619280. J Histochem Cytochem. 1992. PMID: 1619280
-
Distribution pattern and ultrastructural localization of Rxt1, an orphan Na+/Cl(-)-dependent transporter, in the central nervous system of rats and mice.Neuroscience. 1997 Mar;77(2):319-33. doi: 10.1016/s0306-4522(96)00466-6. Neuroscience. 1997. PMID: 9472393
-
Possible involvement of proteasome inhibition in aging: implications for oxidative stress.Mech Ageing Dev. 2000 Jan 24;113(1):61-70. doi: 10.1016/s0047-6374(99)00101-3. Mech Ageing Dev. 2000. PMID: 10708250
-
Intracellular distribution of proteasomes.Curr Opin Immunol. 1998 Feb;10(1):110-4. doi: 10.1016/s0952-7915(98)80040-x. Curr Opin Immunol. 1998. PMID: 9523120 Review.
-
Components of the multicatalytic proteinase complex.Biomed Biochim Acta. 1991;50(4-6):447-50. Biomed Biochim Acta. 1991. PMID: 1724905 Review.
Cited by
-
Dynamic Behavior of the RNA Polymerase II and the Ubiquitin Proteasome System During the Neuronal DNA Damage Response to Ionizing Radiation.Mol Neurobiol. 2016 Dec;53(10):6799-6808. doi: 10.1007/s12035-015-9565-8. Epub 2015 Dec 11. Mol Neurobiol. 2016. PMID: 26660115
-
Differentially expressed lncRNAs and mRNAs identified by microarray analysis in GBS patients vs healthy controls.Sci Rep. 2016 Feb 22;6:21819. doi: 10.1038/srep21819. Sci Rep. 2016. PMID: 26898505 Free PMC article.
-
Neuronal induction of the immunoproteasome in Huntington's disease.J Neurosci. 2003 Dec 17;23(37):11653-61. doi: 10.1523/JNEUROSCI.23-37-11653.2003. J Neurosci. 2003. PMID: 14684867 Free PMC article.
-
In vivo aggregation properties of the nuclear poly(A)-binding protein PABPN1.RNA. 2005 May;11(5):752-62. doi: 10.1261/rna.7217105. Epub 2005 Apr 5. RNA. 2005. PMID: 15811916 Free PMC article.
-
Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy.J Neurosci. 2000 Oct 1;20(19):7268-78. doi: 10.1523/JNEUROSCI.20-19-07268.2000. J Neurosci. 2000. PMID: 11007884 Free PMC article.
References
-
- Arribas J, Arizti P, Castaño JG. Antibodies against the C2 COOH-terminal region discriminate the active and latent forms of the multicatalytic proteinase complex. J Biol Chem. 1994;269:12858–12864. - PubMed
-
- Arribas J, Castaño JG. Kinetic studies of the differential effect of detergents on the peptidase activities of the multicatalytic proteinase from rat liver. J Biol Chem. 1990;265:13969–13973. - PubMed
-
- Arizti P, Arribas J, Castaño J G. Modulation of the multicatalytic proteinase complex by lipids, interconversion and proteolytic processing. Enzyme Protein. 1993;47:285–295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
