Development and postnatal regulation of adult myoblasts
- PMID: 7787236
- PMCID: PMC4082319
- DOI: 10.1002/jemt.1070300504
Development and postnatal regulation of adult myoblasts
Abstract
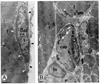
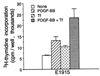
The myogenic precursor cells of postnatal and adult skeletal muscle are situated underneath the basement membrane of the myofibers. It is because of their unique positions that these precursor cells are often referred to as satellite cells. Such defined satellite cells can first be detected following the formation of a distinct basement membrane around the fiber, which takes place in late stages of embryogenesis. Like myoblasts found during development, satellite cells can proliferate, differentiate, and fuse into myofibers. However, in the normal, uninjured adult muscle, satellite cells are mitotically quiescent. In recent years several important questions concerning the biology of satellite cells have been asked. One aspect has been the relationship between satellite cells and myoblasts found in the developing muscle: are these myogenic populations identical or different? Another aspect has been the physiological cues that control the quiescent, proliferative, and differentiative states of these myogenic precursors: what are the growth regulators and how do they function? These issues are discussed, referring to previous work by others and further emphasizing our own studies on avian and rodent satellite cells. Collectively, the studies presented indicate that satellite cells represent a distinct myogenic population that becomes dominant in late stages of embryogenesis. Moreover, although satellite cells are already destined to be myogenic precursors, they do not express any of the four known myogenic regulatory genes unless their activation is induced in the animal or in culture. Furthermore, multiple growth factors are important regulators of satellite cell proliferation and differentiation. Our work on the role of one of these growth factors [platelet-derived growth factor (PDGF)] during proliferation of adult myoblasts is further discussed with greater detail and the possibility that PDGF is involved in the transition from fetal to adult myoblasts in late embryogenesis is brought forward.
Figures






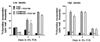
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
"I've Spent My Whole Life Striving to Be Normal": Internalized Stigma and Perceived Impact of Diagnosis in Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):423-436. doi: 10.1089/aut.2022.0066. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116050 Free PMC article.
-
Mapping the scientific knowledge and approaches to defining and measuring hate crime, hate speech, and hate incidents: A systematic review.Campbell Syst Rev. 2024 Apr 28;20(2):e1397. doi: 10.1002/cl2.1397. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38686101 Free PMC article. Review.
Cited by
-
Functional properties of muscle-derived cells related to morphological characteristics.Histochem Cell Biol. 2006 Nov;126(5):603-16. doi: 10.1007/s00418-006-0196-z. Epub 2006 Jun 10. Histochem Cell Biol. 2006. PMID: 16767409
-
Differentiation-dependent PTPIP51 expression in human skeletal muscle cell culture.J Histochem Cytochem. 2009 May;57(5):425-35. doi: 10.1369/jhc.2008.952846. Epub 2009 Jan 5. J Histochem Cytochem. 2009. PMID: 19124842 Free PMC article.
-
Influence of PDGF-BB on proliferation and transition through the MyoD-myogenin-MEF2A expression program during myogenesis in mouse C2 myoblasts.Growth Factors. 1997;15(1):1-27. doi: 10.3109/08977199709002109. Growth Factors. 1997. PMID: 9401815 Free PMC article.
-
Timing Is Everything-The High Sensitivity of Avian Satellite Cells to Thermal Conditions During Embryonic and Posthatch Periods.Front Physiol. 2020 Mar 31;11:235. doi: 10.3389/fphys.2020.00235. eCollection 2020. Front Physiol. 2020. PMID: 32300304 Free PMC article. Review.
-
Peroxisome proliferator-activated receptor beta activation promotes myonuclear accretion in skeletal muscle of adult and aged mice.Pflugers Arch. 2009 Sep;458(5):901-13. doi: 10.1007/s00424-009-0676-9. Epub 2009 May 5. Pflugers Arch. 2009. PMID: 19415321 Free PMC article.
References
-
- Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J. Cell Physiol. 1987;133:567–572. - PubMed
-
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J. Cell Physiol. 1989;138:311–315. - PubMed
-
- Allen RE, Dodson MV, Luiten LS. Regulation of skeletal muscle satellite cell proliferation by bovine pituitary fibroblast growth factor. Exp. Cell. Res. 1984;152:154–160. - PubMed
-
- Allen RE, Rankin LL, Greene EA, Boxhorn LK, Johnson SE, Taylor RG, Pierce PR. Desmin is present in proliferating rat muscle satellite cells but not in bovine muscle satellite cells. J. Cell. Physiol. 1991;149:525–535. - PubMed
-
- Anderson JE, Liu L, Kardami E. Distinctive patterns of bFGF distribution in degenerating and regenerating areas of dystrophic (mdx) striated muscles. Dev. Biol. 1991;147:96–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

