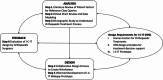
Human-centered design of a health recommender system for orthopaedic shoulder treatment
- PMID: 39794787
- PMCID: PMC11720343
- DOI: 10.1186/s12911-025-02850-x
Human-centered design of a health recommender system for orthopaedic shoulder treatment
Abstract
Background: Rich data on diverse patients and their treatments and outcomes within Electronic Health Record (EHR) systems can be used to generate real world evidence. A health recommender system (HRS) framework can be applied to a decision support system application to generate data summaries for similar patients during the clinical encounter to assist physicians and patients in making evidence-based shared treatment decisions.
Objective: A human-centered design (HCD) process was used to develop a HRS for treatment decision support in orthopaedic medicine, the Informatics Consult for Individualized Treatment (I-C-IT). We also evaluate the usability and utility of the system from the physician's perspective, focusing on elements of utility and shared decision-making in orthopaedic medicine.
Methods: The HCD process for I-C-IT included 6 steps across three phases of analysis, design, and evaluation. A team of health informatics and comparative effectiveness researchers directly engaged with orthopaedic surgeon subject matter experts in a collaborative I-C-IT prototype design process. Ten orthopaedic surgeons participated in a mixed methods evaluation of the I-C-IT prototype that was produced.
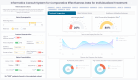
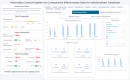
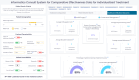
Results: The HCD process resulted in a prototype system, I-C-IT, with 14 data visualization elements and a set of design principles crucial for HRS for decision support. The overall standard system usability scale (SUS) score for the I-C-IT Webapp prototype was 88.75 indicating high usability. In addition, utility questions addressing shared decision-making found that 90% of orthopaedic surgeon respondents either strongly agreed or agreed that I-C-IT would help them make data informed decisions with their patients.
Conclusion: The HCD process produced an HRS prototype that is capable of supporting orthopaedic surgeons and patients in their information needs during clinical encounters. Future research should focus on refining I-C-IT by incorporating patient feedback in future iterative cycles of system design and evaluation.
Keywords: Clinical decision support; Health recommender system; Human factors; Human-centered design; Orthopaedic treatment; Personalized medicine; Proximal humerus fracture.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This final study protocol was reviewed and approved by the Institutional Review Board at Prisma Health. The IRB waived the requirement of signed informed consent, but physician participants were provided an information sheet about the study and were allowed to decline enrollment in the I-C-IT Evaluation. Their verbal agreement to continue with the interview served as consent to participate. All methods were carried out in accordance with relevant guidelines and regulations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Conflict of interest: None to disclose.
Figures
Update of
-
Human-centered Design of a Health Recommender System for Orthopaedic Shoulder Treatment.Res Sq [Preprint]. 2024 May 21:rs.3.rs-4359437. doi: 10.21203/rs.3.rs-4359437/v1. Res Sq. 2024. Update in: BMC Med Inform Decis Mak. 2025 Jan 10;25(1):17. doi: 10.1186/s12911-025-02850-x. PMID: 38826294 Free PMC article. Updated. Preprint.
Similar articles
-
Human-centered Design of a Health Recommender System for Orthopaedic Shoulder Treatment.Res Sq [Preprint]. 2024 May 21:rs.3.rs-4359437. doi: 10.21203/rs.3.rs-4359437/v1. Res Sq. 2024. Update in: BMC Med Inform Decis Mak. 2025 Jan 10;25(1):17. doi: 10.1186/s12911-025-02850-x. PMID: 38826294 Free PMC article. Updated. Preprint.
-
Interventions for supporting pregnant women's decision-making about mode of birth after a caesarean.Cochrane Database Syst Rev. 2013 Jul 30;2013(7):CD010041. doi: 10.1002/14651858.CD010041.pub2. Cochrane Database Syst Rev. 2013. PMID: 23897547 Free PMC article. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Can We Enhance Shared Decision-making for Periacetabular Osteotomy Surgery? A Qualitative Study of Patient Experiences.Clin Orthop Relat Res. 2025 Jan 1;483(1):120-136. doi: 10.1097/CORR.0000000000003198. Epub 2024 Jul 23. Clin Orthop Relat Res. 2025. PMID: 39051876
-
Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures.Cochrane Database Syst Rev. 2013 Jul 6;2013(7):CD009445. doi: 10.1002/14651858.CD009445.pub2. Cochrane Database Syst Rev. 2013. PMID: 23832767 Free PMC article. Review.
References
-
- Sheridan DJ, Julian DG. Achievements and limitations of evidence-based medicine. J Am Coll Cardiol. 2016;68(2):204–13. - PubMed
-
- Brooks JM, Chrischilles EA. Heterogeneity and the interpretation of treatment effect estimates from risk adjustment and instrumental variable methods. Med Care [Internet]. 2007 Oct [cited 2020 Mar 22];45(10 Supl 2):S123-30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17909370 - PubMed
-
- Brooks JM, Chapman CG, Schroeder MC. Understanding Treatment Effect Estimates When Treatment Effects Are Heterogeneous for More Than One Outcome. Appl Health Econ Health Policy [Internet]. 2018 Jun 1 [cited 2020 Mar 22];16(3):381–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29589296 - PMC - PubMed
-
- Angrist JD. Treatment Effect Heterogeneity in Theory and Practice. The Economic Journal [Internet]. 2004 Mar 1 [cited 2023 Nov 27];114(494):C52–83. Available from: 10.1111/j.0013-0133.2003.00195.x
MeSH terms
LinkOut - more resources
Full Text Sources