Interneuron FGF13 regulates seizure susceptibility via a sodium channel-independent mechanism
- PMID: 39773461
- PMCID: PMC11709433
- DOI: 10.7554/eLife.98661
Interneuron FGF13 regulates seizure susceptibility via a sodium channel-independent mechanism
Abstract
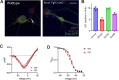
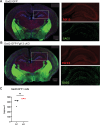
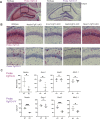
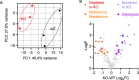
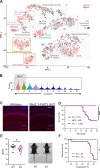
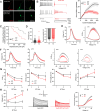
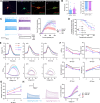
Developmental and epileptic encephalopathies (DEEs), a class of devastating neurological disorders characterized by recurrent seizures and exacerbated by disruptions to excitatory/inhibitory balance in the brain, are commonly caused by mutations in ion channels. Disruption of, or variants in, FGF13 were implicated as causal for a set of DEEs, but the underlying mechanisms were clouded because FGF13 is expressed in both excitatory and inhibitory neurons, FGF13 undergoes extensive alternative splicing producing multiple isoforms with distinct functions, and the overall roles of FGF13 in neurons are incompletely cataloged. To overcome these challenges, we generated a set of novel cell-type-specific conditional knockout mice. Interneuron-targeted deletion of Fgf13 led to perinatal mortality associated with extensive seizures and impaired the hippocampal inhibitory/excitatory balance while excitatory neuron-targeted deletion of Fgf13 caused no detectable seizures and no survival deficits. While best studied as a voltage-gated sodium channel (Nav) regulator, we observed no effect of Fgf13 ablation in interneurons on Navs but rather a marked reduction in K+ channel currents. Re-expressing different Fgf13 splice isoforms could partially rescue deficits in interneuron excitability and restore K+ channel current amplitude. These results enhance our understanding of the molecular mechanisms that drive the pathogenesis of Fgf13-related seizures and expand our understanding of FGF13 functions in different neuron subsets.
Keywords: epilepsy; inhibitory interneuron; mouse; neuroscience; sodium channels.
© 2024, Lin, Gade et al.
Conflict of interest statement
SL, AG, HW, JN, AG, ID, PT, JN, MM, TS, AR No competing interests declared, GP is a scientific advisory board member for Tevard Biosciences
Figures







Update of
-
Interneuron FGF13 regulates seizure susceptibility via a sodium channel-independent mechanism.bioRxiv [Preprint]. 2024 Aug 19:2024.04.18.590019. doi: 10.1101/2024.04.18.590019. bioRxiv. 2024. Update in: Elife. 2025 Jan 08;13:RP98661. doi: 10.7554/eLife.98661 PMID: 38659789 Free PMC article. Updated. Preprint.
Similar articles
-
Interneuron FGF13 regulates seizure susceptibility via a sodium channel-independent mechanism.bioRxiv [Preprint]. 2024 Aug 19:2024.04.18.590019. doi: 10.1101/2024.04.18.590019. bioRxiv. 2024. Update in: Elife. 2025 Jan 08;13:RP98661. doi: 10.7554/eLife.98661 PMID: 38659789 Free PMC article. Updated. Preprint.
-
Lamotrigine versus levetiracetam or zonisamide for focal epilepsy and valproate versus levetiracetam for generalised and unclassified epilepsy: two SANAD II non-inferiority RCTs.Health Technol Assess. 2021 Dec;25(75):1-134. doi: 10.3310/hta25750. Health Technol Assess. 2021. PMID: 34931602 Clinical Trial.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Multiple layers of diversity govern the cell type specificity of GABAergic input received by mouse subicular pyramidal neurons.J Physiol. 2024 Sep;602(17):4195-4213. doi: 10.1113/JP286679. Epub 2024 Aug 14. J Physiol. 2024. PMID: 39141819 Free PMC article.
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
References
-
- Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW. GeneReviews. National Library of Medicine; 1993.
-
- Brunklaus A, Pérez-Palma E, Ghanty I, Xinge J, Brilstra E, Ceulemans B, Chemaly N, Depienne C, Guerrini R, Mei D, Møller RS, Nabbout R, Regan BM, Schneider AL, Scheffer IE, Schoonjans AS, Symonds JD, Weckhuysen S, Kattan MW, Zuberi SM, Lal D. Development and validation of a prediction model for early diagnosis of. Neurology. 2022;98:e1163–e1174. doi: 10.1212/wnl.0000000000200028. - DOI - PMC - PubMed
-
- Bublik DR, Bursać S, Sheffer M, Oršolić I, Shalit T, Tarcic O, Kotler E, Mouhadeb O, Hoffman Y, Fuchs G, Levin Y, Volarević S, Oren M. Regulatory module involving FGF13, miR-504, and p53 regulates ribosomal biogenesis and supports cancer cell survival. PNAS. 2017;114:E496–E505. doi: 10.1073/pnas.1614876114. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

