Naturally acquired IgG responses to Plasmodium falciparum do not target the conserved termini of the malaria vaccine candidate Merozoite Surface Protein 2
- PMID: 39717775
- PMCID: PMC11663719
- DOI: 10.3389/fimmu.2024.1501700
Naturally acquired IgG responses to Plasmodium falciparum do not target the conserved termini of the malaria vaccine candidate Merozoite Surface Protein 2
Abstract
Introduction: Malaria remains a significant burden, and a fully protective vaccine against Plasmodium falciparum is critical for reducing morbidity and mortality. Antibody responses against the blood-stage antigen Merozoite Surface Protein 2 (MSP2) are associated with protection from P. falciparum malaria, but its extensive polymorphism is a barrier to its development as a vaccine candidate. New tools, such as long-read sequencing and accurate protein structure modelling allow us to study the genetic diversity and immune responses towards antigens from clinical isolates with unprecedented detail. This study sought to better understand naturally acquired MSP2-specific antibody responses.
Methods: IgG responses against recombinantly expressed full-length, central polymorphic regions, and peptides derived from the conserved termini of MSP2 variants sequenced from patient isolates, were tested in plasma from travelers with recent, acute malaria and from individuals living in an endemic area of Tanzania.
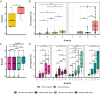
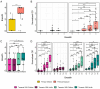
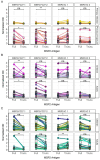
Results: IgG responses towards full MSP2 and truncated MSP2 antigens were variant specific. IgG antibodies in the plasma of first-time infected or previously exposed travelers did not recognize the conserved termini of expressed MSP2 variants by ELISA, but they bound 13-amino acid long linear epitopes from the termini in a custom-made peptide array. Alphafold3 modelling suggests extensive structural heterogeneity in the conserved termini upon antigen oligomerization. IgG from individuals living in an endemic region, many who were asymptomatically infected, did not recognize the conserved termini by ELISA.
Discussion: Our results suggest that responses to the variable regions are critical for the development of naturally acquired immunity towards MSP2.
Keywords: antibody response; immune evasion; malaria vaccine; merozoite; natural immunity; polymorphic antigens; structural heterogeneity.
Copyright © 2024 Zerebinski, Margerie, Han, Moll, Ritvos, Jahnmatz, Ahlborg, Ngasala, Rooth, Sjöberg, Sundling, Yman, Färnert and Plaza.
Conflict of interest statement
PJ and NA were employed by MabTech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





Similar articles
-
Estimation of PfRh5-based vaccine efficacy in asymptomatic Plasmodium falciparum patients from high-endemic areas of Tanzania using genetic and antigenicity variation screening.Front Immunol. 2024 Nov 18;15:1495513. doi: 10.3389/fimmu.2024.1495513. eCollection 2024. Front Immunol. 2024. PMID: 39624090 Free PMC article.
-
Plasmodium Falciparum and mosquito vector IgG patterns across suspected malaria cases in Ghana.BMC Infect Dis. 2024 Dec 2;24(1):1374. doi: 10.1186/s12879-024-10248-9. BMC Infect Dis. 2024. PMID: 39623362 Free PMC article.
-
Human Immunization With a Polymorphic Malaria Vaccine Candidate Induced Antibodies to Conserved Epitopes That Promote Functional Antibodies to Multiple Parasite Strains.J Infect Dis. 2018 Jun 5;218(1):35-43. doi: 10.1093/infdis/jiy170. J Infect Dis. 2018. PMID: 29584918 Free PMC article. Clinical Trial.
-
Vaccine candidate discovery for the next generation of malaria vaccines.Immunology. 2017 Oct;152(2):195-206. doi: 10.1111/imm.12780. Epub 2017 Jul 24. Immunology. 2017. PMID: 28646586 Free PMC article. Review.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
References
-
- Gilson PR, Nebl T, Vukcevic D, Moritz RL, Sargeant T, Speed TP, et al. . Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. (2006) 5:1286–99. doi: 10.1074/MCP.M600035-MCP200 - DOI - PubMed
-
- World malaria report. Geneva: World Health Organization; (2023).
-
- Datoo MS, Natama MH, Somé A, Traoré O, Rouamba T, Bellamy D, et al. . Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomized controlled trial. Lancet. (2021) 397:1809–18. doi: 10.1016/S0140-6736(21)00943-0 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

