Brain-derived and in vitro-seeded alpha-synuclein fibrils exhibit distinct biophysical profiles
- PMID: 39584804
- PMCID: PMC11588339
- DOI: 10.7554/eLife.92775
Brain-derived and in vitro-seeded alpha-synuclein fibrils exhibit distinct biophysical profiles
Abstract
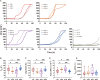
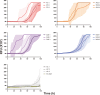
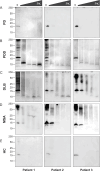
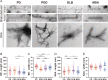
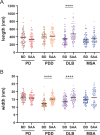
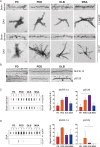
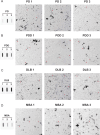
The alpha-synuclein (αSyn) seeding amplification assay (SAA) that allows the generation of disease-specific in vitro seeded fibrils (SAA fibrils) is used as a research tool to study the connection between the structure of αSyn fibrils, cellular seeding/spreading, and the clinicopathological manifestations of different synucleinopathies. However, structural differences between human brain-derived and SAA αSyn fibrils have been recently highlighted. Here, we characterize the biophysical properties of the human brain-derived αSyn fibrils from the brains of patients with Parkinson's disease with and without dementia (PD, PDD), dementia with Lewy bodies (DLB), multiple system atrophy (MSA), and compare them to the 'model' SAA fibrils. We report that the brain-derived αSyn fibrils show distinct biochemical profiles, which were not replicated in the corresponding SAA fibrils. Furthermore, the brain-derived αSyn fibrils from all synucleinopathies displayed a mixture of 'straight' and 'twisted' microscopic structures. However, the PD, PDD, and DLB SAA fibrils had a 'straight' structure, whereas MSA SAA fibrils showed a 'twisted' structure. Finally, the brain-derived αSyn fibrils from all four synucleinopathies were phosphorylated (S129). Interestingly, phosphorylated αSyn were carried over to the PDD and DLB SAA fibrils. Our findings demonstrate the limitation of the SAA fibrils modeling the brain-derived αSyn fibrils and pay attention to the necessity of deepening the understanding of the SAA fibrillation methodology.
Keywords: Alpha-synuclein; Synucleinopathies; human; neuroscience; seeded amplification assay; strains.
Plain language summary
Alpha-synuclein is a protein that is essential for brain function. Like all proteins, alpha-synuclein is made of a string of amino acids and folded up in a precise way. Misfolding of this protein can lead to a buildup of protein clumps in the brain. These clumps cause neurodegenerative diseases like Parkinson’s disease, Parkinson’s disease with dementia, dementia with Lewy bodies, and multiple system atrophy. Each of these diseases affects different parts of the brain and has distinct symptoms. Studying the misfolding patterns and structures of proteins collected from the brains of deceased people who had these conditions may help scientists understand the differences. Scientists also try to recreate these misfolded proteins in the laboratory using a seeding amplification assay technique. This is important as scientists can only extract limited amounts of protein clumps from human brains. However, it is unclear if the laboratory-produced protein clumps behave exactly like brain-derived clumps. Learning more is essential to make sure that studies of these proteins produce accurate results. Lee et al. identified differences between alpha-synuclein clumps extracted from patients with different brain diseases. They also show that laboratory-derived protein clumps do not exactly recreate those from patients. Clumps from patients with Parkinson’s disease, Parkinson’s disease with dementia, dementia with Lewy bodies, and multiple system atrophy have straight and twisted protein structures when examined under an electron microscope. But the laboratory-generated clumps for Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies are all straight. Laboratory-generated clumps for multiple system atrophy are all twisted. The clumps from patient brains also have many phosphate groups attached to them. Laboratory-derived clumps of Parkinson’s disease with dementia and dementia with Lewy bodies also had this feature. However, the lab-derived versions of the other disease clumps lack this characteristic. Lee et al. identify essential differences between the protein clumps from the brains of patients with different neurodegenerative diseases. They also found that the laboratory-generated clumps do not completely replicate all their features. More research is needed on these disease-specific differences and how they contribute to specific disease symptoms and disease progress. Work is also required to refine laboratory processes for protein clumps to replicate disease-associated clumps better. More studies could help scientists develop better laboratory models for these diseases that can be used to create and test new therapies.
© 2024, Lee et al.
Conflict of interest statement
SL, LC, LP No competing interests declared
Figures




Update of
- doi: 10.1101/2023.10.04.560803
- doi: 10.7554/eLife.92775.1
- doi: 10.7554/eLife.92775.2
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
"I've Spent My Whole Life Striving to Be Normal": Internalized Stigma and Perceived Impact of Diagnosis in Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):423-436. doi: 10.1089/aut.2022.0066. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116050 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
References
-
- Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovács GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H. Staging of neurofibrillary pathology in Alzheimer’s disease: A study of the BrainNet Europe Consortium. Brain Pathology. 2008;18:484–496. doi: 10.1111/j.1750-3639.2008.00147.x. - DOI - PMC - PubMed
-
- Alafuzoff I, Ince PG, Arzberger T, Al-Sarraj S, Bell J, Bodi I, Bogdanovic N, Bugiani O, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Ironside JW, Kavantzas N, King A, Korkolopoulou P, Kovács GG, Meyronet D, Monoranu C, Parchi P, Parkkinen L, Patsouris E, Roggendorf W, Rozemuller A, Stadelmann-Nessler C, Streichenberger N, Thal DR, Kretzschmar H. Staging/typing of Lewy body related alpha-synuclein pathology: A study of the BrainNet Europe Consortium. Acta Neuropathologica. 2009;117:635–652. doi: 10.1007/s00401-009-0523-2. - DOI - PubMed
-
- Balana AT, Mahul-Mellier AL, Nguyen BA, Horvath M, Javed A, Hard ER, Jasiqi Y, Singh P, Afrin S, Pedretti R, Singh V, Lee VMY, Luk KC, Saelices L, Lashuel HA, Pratt MR. O-GlcNAc Modification Forces the Formation of an α-Synuclein Amyloid-Strain with Notably Diminished Seeding Activity and Pathology. bioRxiv. 2023 doi: 10.1101/2023.03.07.531573. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

