Characteristics of non-randomised studies of drug treatments: cross sectional study
- PMID: 39574419
- PMCID: PMC11579539
- DOI: 10.1136/bmjmed-2024-000932
Characteristics of non-randomised studies of drug treatments: cross sectional study
Abstract
Objective: To examine the characteristics of comparative non-randomised studies that assess the effectiveness or safety, or both, of drug treatments.
Design: Cross sectional study.
Data sources: Medline (Ovid), for reports published from 1 June 2022 to 31 August 2022.
Eligibility criteria for selecting studies: Reports of comparative non-randomised studies that assessed the effectiveness or safety, or both, of drug treatments were included. A randomly ordered sample was screened until 200 eligible reports were found. Data on general characteristics, reporting characteristics, and time point alignment were extracted, and possible related biases, with a piloted form inspired by reporting guidelines and the target trial emulation framework.
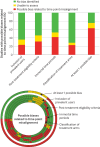
Results: Of 462 reports of non-randomised studies identified, 262 studies were excluded (32% had no comparator and 25% did not account for confounding factors). To assess time point alignment and possible related biases, three study time points were considered: eligibility, treatment assignment, and start of follow-up. Of the 200 included reports, 70% had one possible bias, related to: inclusion of prevalent users in 24%, post-treatment eligibility criteria in 32%, immortal time periods in 42%, and classification of treatment in 23%. Reporting was incomplete, and only 2% reported all six of the key elements considered: eligibility criteria (87%), description of treatment (46%), deviations in treatment (27%), causal contrast (11%), primary outcomes (90%), and confounding factors (88%). Most studies used routinely collected data (67%), but only 7% reported using validation studies of the codes or algorithms applied to select the population. Only 7% of reports mentioned registration on a trial registry and 3% had an available protocol.
Conclusions: The findings of the study suggest that although access to real world evidence could be valuable, the robustness and transparency of non-randomised studies need to be improved.
Keywords: Drug therapy; Epidemiology; Research design.
Copyright © Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
All authors have completed the ICMJE uniform disclose form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Association between pacifier use and breast-feeding, sudden infant death syndrome, infection and dental malocclusion.JBI Libr Syst Rev. 2005;3(6):1-33. doi: 10.11124/01938924-200503060-00001. JBI Libr Syst Rev. 2005. PMID: 27819973
-
Recovery schools for improving behavioral and academic outcomes among students in recovery from substance use disorders: a systematic review.Campbell Syst Rev. 2018 Oct 4;14(1):1-86. doi: 10.4073/csr.2018.9. eCollection 2018. Campbell Syst Rev. 2018. PMID: 37131375 Free PMC article.
-
The epidemiology, management and impact of surgical wounds healing by secondary intention: a research programme including the SWHSI feasibility RCT.Southampton (UK): NIHR Journals Library; 2020 Sep. Southampton (UK): NIHR Journals Library; 2020 Sep. PMID: 32960518 Free Books & Documents. Review.
-
Individual-level interventions to reduce personal exposure to outdoor air pollution and their effects on people with long-term respiratory conditions.Cochrane Database Syst Rev. 2021 Aug 9;8(8):CD013441. doi: 10.1002/14651858.CD013441.pub2. Cochrane Database Syst Rev. 2021. PMID: 34368949 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
