Cellular and molecular roles of reactive oxygen species in wound healing
- PMID: 39562800
- PMCID: PMC11577046
- DOI: 10.1038/s42003-024-07219-w
Cellular and molecular roles of reactive oxygen species in wound healing
Abstract
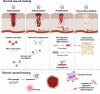
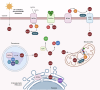
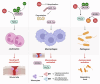
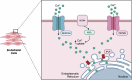
Wound healing is a highly coordinated spatiotemporal sequence of events involving several cell types and tissues. The process of wound healing requires strict regulation, and its disruption can lead to the formation of chronic wounds, which can have a significant impact on an individual's health as well as on worldwide healthcare expenditure. One essential aspect within the cellular and molecular regulation of wound healing pathogenesis is that of reactive oxygen species (ROS) and oxidative stress. Wounding significantly elevates levels of ROS, and an array of various reactive species are involved in modulating the wound healing process, such as through antimicrobial activities and signal transduction. However, as in many pathologies, ROS play an antagonistic pleiotropic role in wound healing, and can be a pathogenic factor in the formation of chronic wounds. Whilst advances in targeting ROS and oxidative stress have led to the development of novel pre-clinical therapeutic methods, due to the complex nature of ROS in wound healing, gaps in knowledge remain concerning the specific cellular and molecular functions of ROS in wound healing. In this review, we highlight current knowledge of these functions, and discuss the potential future direction of new studies, and how these pathways may be targeted in future pre-clinical studies.
© 2024. The Author(s).
Conflict of interest statement
Figures
Similar articles
-
Reactive Oxygen Species and NOX Enzymes Are Emerging as Key Players in Cutaneous Wound Repair.Int J Mol Sci. 2017 Oct 15;18(10):2149. doi: 10.3390/ijms18102149. Int J Mol Sci. 2017. PMID: 29036938 Free PMC article. Review.
-
M1 macrophage mediated increased reactive oxygen species (ROS) influence wound healing via the MAPK signaling in vitro and in vivo.Toxicol Appl Pharmacol. 2019 Mar 1;366:83-95. doi: 10.1016/j.taap.2019.01.022. Epub 2019 Jan 25. Toxicol Appl Pharmacol. 2019. PMID: 30690042
-
Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process.Int Wound J. 2017 Feb;14(1):89-96. doi: 10.1111/iwj.12557. Epub 2015 Dec 21. Int Wound J. 2017. PMID: 26688157 Free PMC article.
-
ROS-scavenging materials for skin wound healing: advancements and applications.Front Bioeng Biotechnol. 2023 Dec 12;11:1304835. doi: 10.3389/fbioe.2023.1304835. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38149175 Free PMC article. Review.
-
Revisiting the essential role of oxygen in wound healing.Am J Surg. 2003 Sep;186(3):259-63. doi: 10.1016/s0002-9610(03)00211-3. Am J Surg. 2003. PMID: 12946829 Review.
References
-
- Peña, O. & Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 25, 599–616 (2024). - PubMed
-
- Torres, M. et al. The temporal dynamics of proteins in aged skin wound healing and comparison to gene expression. J. Invest. Dermatol. (2024).
-
- Schultz, G. et al. In Principles of Wound Healing. (University of Adelaide Press, 2011). - PubMed
-
- Eming, S., Krieg, T. & Davidson, J. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol.127, 514–525 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

