Efgartigimod versus intravenous immunoglobulin in the treatment of patients with impending myasthenic crisis
- PMID: 39551862
- PMCID: PMC11570634
- DOI: 10.1038/s41598-024-79918-7
Efgartigimod versus intravenous immunoglobulin in the treatment of patients with impending myasthenic crisis
Abstract
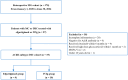
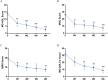
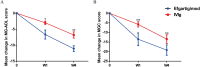
Impending myasthenic crisis (IMC) is an emergent situation requiring aggressive management to prevent patients from developing myasthenic crisis (MC) in patients with myasthenia gravis (MG). Efgartigimod has been proved to be well tolerated and efficacious in MG patients. The present study aimed to compare the efficacy of efgartigimod and intravenous immunoglobulin (IVIg) in rescuing IMC. IMC patients treated with efgartigimod or IVIg were retrospectively enrolled. The primary outcome was determined as the mean change in MG activities of daily living (MG-ADL) score from baseline to week 1 and 4 after treatment, respectively. Safety was assessed based on medical records during the hospitalization to monitor the adverse events. A total of 9 patients treated with efgartigimod and 10 patients treated with IVIg were enrolled. There were no significant differences in the clinical characteristics at baseline between the two groups (P > 0.05). Compared with the IVIg group, the efgartigimod group had a greater reduction in the MG-ADL score at week 1 (P = 0.035) and week 4 (P = 0.005). One patient in the efgartigimod group had an upper respiratory infection. These findings suggest that efgartigimod is a treatment option for IMC in addition to IVIg and plasma exchange.
Keywords: Efficacy; Efgartigimod; IVIg; Impending myasthenic crisis; Myasthenia gravis.
© 2024. The Author(s).
Conflict of interest statement
Figures
Similar articles
-
Efgartigimod as a fast-acting add-on therapy in manifest and impending myasthenic crisis: A single-center case series.J Neuroimmunol. 2024 Oct 15;395:578431. doi: 10.1016/j.jneuroim.2024.578431. Epub 2024 Aug 10. J Neuroimmunol. 2024. PMID: 39142025
-
Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial.Lancet Neurol. 2021 Jul;20(7):526-536. doi: 10.1016/S1474-4422(21)00159-9. Lancet Neurol. 2021. PMID: 34146511 Clinical Trial.
-
Efgartigimod combined with steroids as a fast-acting therapy for myasthenic crisis: a case report.BMC Neurol. 2024 Aug 22;24(1):292. doi: 10.1186/s12883-024-03804-y. BMC Neurol. 2024. PMID: 39174898 Free PMC article.
-
Single institution experience with efgartigimod in patients with myasthenia gravis: Patient selection, dosing schedules, treatment response, and adverse events.Muscle Nerve. 2024 Jan;69(1):87-92. doi: 10.1002/mus.28003. Epub 2023 Nov 21. Muscle Nerve. 2024. PMID: 37990374 Review.
-
Retrospective review of patients with myasthenia gravis switched from plasma exchange therapy to efgartigimod treatment.Muscle Nerve. 2024 Apr;69(4):467-471. doi: 10.1002/mus.28042. Epub 2024 Jan 29. Muscle Nerve. 2024. PMID: 38284651 Review.
References
-
- Nils Erik, G. & Jan, V. J. Myasthenia gravis: Subgroup classification and therapeutic strategies. Lancet Neurol.10.1016/s1474-4422(15)00145-3 (2015). - PubMed
-
- Rui, Z., Sushan, L. & Chongbo, Z. The role of innate immunity in myasthenia gravis. Autoimmun. Rev.10.1016/j.autrev.2021.102800 (2021). - PubMed
-
- Osamu, H. et al. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann. Neurol.10.1002/ana.22312 (2011). - PubMed
-
- James, F. H. Jr. et al. Clinical effects of the self-administered subcutaneous complement inhibitor zilucoplan in patients with moderate to severe generalized myasthenia gravis: Results of a phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. JAMA Neurol.10.1001/jamaneurol.2019.5125 (2020). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

