The heterologous expression of novel recombinant protein composed of HN and F moieties of Newcastle disease virus and immunogenicity evaluation in mouse model
- PMID: 39534294
- PMCID: PMC11551657
- DOI: 10.18502/ijm.v16i5.16801
The heterologous expression of novel recombinant protein composed of HN and F moieties of Newcastle disease virus and immunogenicity evaluation in mouse model
Abstract
Background and objectives: The rapid spread of Newcastle disease (ND), driven by extensive commercial exchange in the poultry industry, necessitates urgent preventive measures. Although effective vaccines against the Newcastle disease virus (NDV) have been used since 1940, recent outbreaks and the limitations of current vaccines highlight the need for improved solutions. Advances in synthetic biology, reverse vaccinology, molecular biology, and recombinant DNA technology over the past 20 years have led to the development of recombinant vaccines, which offer enhanced protection and broader immunogenic coverage against NDV. This study aimed to express the immunogenic domains of Hemagglutinin Neuraminidase (HN) and Fusion (F) glycoproteins, linked to the heat-labile enterotoxin B subunit (LTB) bio-adjuvant, to develop an effective and reliable recombinant vaccine for NDV.
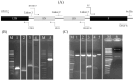
Materials and methods: In this study, the L(HN)2F protein, composed of the LTB bio-adjuvant and the immunogenic regions of the doubled Hemagglutinin Neuraminidase (HN-HN) and Fusion (F) epitope, was expressed in Escherichia coli. Subcutaneous injection was used to evaluate the humoral immune response in mice and the result was compared with B1 vaccine.
Results: The induction of strong humoral immune responses proved the strong immunoreactivity of the recombinant protein.
Conclusion: The IgG elicited by the recombinant proteins was comparable to that of the commercial B1 vaccine against NDV, indicating its potential as a viable candidate for further development and evaluation as a recombinant vaccine against NDV.
Keywords: Fusion (F) glycoprotein; Hemagglutinin neuraminidase; Immune response; LTB bio-adjuvant; Newcastle disease.
Copyright© 2024 The Authors. Published by Tehran University of Medical Sciences.
Conflict of interest statement
Conflict of interest. Atena Mozafari, Mehregan Rahmani, Yasaman Yasini Nasab, Shahla Shahsavandi, Mahyat Jafari and Ali Hatef Salmanian declare that they have no conflicts of interest.
Figures


Similar articles
-
Simultaneous Expression of Chicken Granulocyte Monocyte Colony-Stimulating Factor and the Hemagglutinin-Neuraminidase Epitope of the Virulent Newcastle Disease Virus Genotype VII C22 Strain in a Functional Synthetic Recombinant Adenovirus as a Genotype-Matched Vaccine with Potential Antiviral Activity.Microbiol Spectr. 2023 Jun 15;11(3):e0402422. doi: 10.1128/spectrum.04024-22. Epub 2023 Apr 10. Microbiol Spectr. 2023. PMID: 37036344 Free PMC article.
-
The heat-labile enterotoxin B subunit bio-adjuvant linked to Newcastle disease virus recombinant hemagglutinin neuraminidase elicited a humoral immune response in animal model.Cell Mol Biol (Noisy-le-grand). 2023 Oct 31;69(10):94-99. doi: 10.14715/cmb/2023.69.10.13. Cell Mol Biol (Noisy-le-grand). 2023. PMID: 37953579
-
Recombinant infectious bronchitis virus (IBV) H120 vaccine strain expressing the hemagglutinin-neuraminidase (HN) protein of Newcastle disease virus (NDV) protects chickens against IBV and NDV challenge.Arch Virol. 2016 May;161(5):1209-16. doi: 10.1007/s00705-016-2764-4. Epub 2016 Feb 12. Arch Virol. 2016. PMID: 26873815 Free PMC article.
-
Induction of Immune Response in Animal Model Using Recombinant Anti-NDV Vaccine.Iran J Biotechnol. 2019 Jan 11;17(1):e2215. doi: 10.21859/ijb.2215. eCollection 2019 Jan. Iran J Biotechnol. 2019. PMID: 31457048 Free PMC article.
-
Molecular characterization of hemagglutinin-neuraminidase fragment gene of Newcastle disease virus isolated from periodically-vaccinated farms.Vet World. 2018 May;11(5):657-666. doi: 10.14202/vetworld.2018.657-666. Epub 2018 May 20. Vet World. 2018. PMID: 29915505 Free PMC article. Review.
References
-
- Tan SW, Ideris A, Omar AR, Yusoff K, Hair-Bejo M. Sequence and phylogenetic analysis of Newcastle disease virus genotypes isolated in Malaysia between 2004 and 2005. Arch Virol 2010; 155: 63–70. - PubMed
-
- Bello MB, Mahamud SNA, Yusoff K, Ideris A, Hair-Bejo M, Peeters BPH, et al. Development of an effective and stable genotype-matched live attenuated Newcastle disease virus vaccine based on a novel naturally recombinant Malaysian isolate using reverse genetics. Vaccines (Basel) 2020; 8: 270. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
