RIPK2 Is Crucial for the Microglial Inflammatory Response to Bacterial Muramyl Dipeptide but Not to Lipopolysaccharide
- PMID: 39519307
- PMCID: PMC11546996
- DOI: 10.3390/ijms252111754
RIPK2 Is Crucial for the Microglial Inflammatory Response to Bacterial Muramyl Dipeptide but Not to Lipopolysaccharide
Abstract
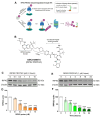
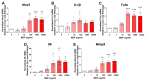
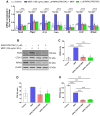
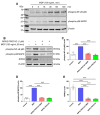
Receptor-interacting serine/threonine protein kinase 2 (RIPK2) is a kinase that is essential in modulating innate and adaptive immune responses. As a downstream signaling molecule for nucleotide-binding oligomerization domain 1 (NOD1), NOD2, and Toll-like receptors (TLRs), it is implicated in the signaling triggered by recognition of microbe-associated molecular patterns by NOD1/2 and TLRs. Upon activation of these innate immune receptors, RIPK2 mediates the release of pro-inflammatory factors by activating mitogen-activated protein kinases (MAPKs) and nuclear factor-kappa B (NF-κB). However, whether RIPK2 is essential for downstream inflammatory signaling following the activation of NOD1/2, TLRs, or both remains controversial. In this study, we examined the role of RIPK2 in NOD2- and TLR4-dependent signaling cascades following stimulation of microglial cells with bacterial muramyl dipeptide (MDP), a NOD2 agonist, or lipopolysaccharide (LPS), a TLR4 agonist. We utilized a highly specific proteolysis targeting chimera (PROTAC) molecule, GSK3728857A, and found dramatic degradation of RIPK2 in a concentration- and time-dependent manner. Importantly, the PROTAC completely abolished MDP-induced increases in iNOS and COX-2 protein levels and pro-inflammatory gene transcription of Nos2, Ptgs2, Il-1β, Tnfα, Il6, Ccl2, and Mmp9. However, increases in iNOS and COX-2 proteins and pro-inflammatory gene transcription induced by the TLR4 agonist, LPS, were only slightly attenuated with the GSK3728857A pretreatment. Further findings revealed that the RIPK2 PROTAC completely blocked the phosphorylation and activation of p65 NF-κB and p38 MAPK induced by MDP, but it had no effects on the phosphorylation of these two mediators triggered by LPS. Collectively, our findings strongly suggest that RIPK2 plays an essential role in the inflammatory responses of microglia to bacterial MDP but not to LPS.
Keywords: Toll-like receptor; inflammatory response; lipopolysaccharide; microglia; mitogen-activated protein kinase; muramyl dipeptide; nuclear factor-kappa B; nucleotide-binding oligomerization domain-like receptor; receptor-interacting serine/threonine protein kinase 2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Update of
-
RIPK2 is crucial for the microglial inflammatory response to bacterial muramyl dipeptide but not to lipopolysaccharide.bioRxiv [Preprint]. 2024 Oct 12:2024.10.09.617444. doi: 10.1101/2024.10.09.617444. bioRxiv. 2024. Update in: Int J Mol Sci. 2024 Nov 01;25(21):11754. doi: 10.3390/ijms252111754 PMID: 39416057 Free PMC article. Updated. Preprint.
Similar articles
-
RIPK2 is crucial for the microglial inflammatory response to bacterial muramyl dipeptide but not to lipopolysaccharide.bioRxiv [Preprint]. 2024 Oct 12:2024.10.09.617444. doi: 10.1101/2024.10.09.617444. bioRxiv. 2024. Update in: Int J Mol Sci. 2024 Nov 01;25(21):11754. doi: 10.3390/ijms252111754 PMID: 39416057 Free PMC article. Updated. Preprint.
-
Nod2 and Rip2 contribute to innate immune responses in mouse neutrophils.Immunology. 2014 Oct;143(2):269-76. doi: 10.1111/imm.12307. Immunology. 2014. PMID: 24766550 Free PMC article.
-
MDP-Induced selective tolerance to TLR4 ligands: impairment in NOD2 mutant Crohn's disease patients.Inflamm Bowel Dis. 2009 Nov;15(11):1686-96. doi: 10.1002/ibd.21013. Inflamm Bowel Dis. 2009. PMID: 19572373
-
Receptor Interacting Ser/Thr-Protein Kinase 2 as a New Therapeutic Target.J Med Chem. 2023 Nov 9;66(21):14391-14410. doi: 10.1021/acs.jmedchem.3c00593. Epub 2023 Oct 19. J Med Chem. 2023. PMID: 37857324 Review.
-
[Bone destruction caused by osteoclasts].Clin Calcium. 2006 Feb;16(2):234-40. Clin Calcium. 2006. PMID: 16465024 Review. Japanese.
References
-
- Wicherska-Pawlowska K., Wrobel T., Rybka J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int. J. Mol. Sci. 2021;22:13397. doi: 10.3390/ijms222413397. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

