Recent Insights into Endogenous Mammalian Cardiac Regeneration Post-Myocardial Infarction
- PMID: 39519298
- PMCID: PMC11546116
- DOI: 10.3390/ijms252111747
Recent Insights into Endogenous Mammalian Cardiac Regeneration Post-Myocardial Infarction
Abstract
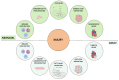
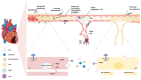
Myocardial infarction (MI) is a critical global health issue and a leading cause of heart failure. Indeed, while neonatal mammals can regenerate cardiac tissue mainly through cardiomyocyte proliferation, this ability is lost shortly after birth, resulting in the adult heart's inability to regenerate after injury effectively. In adult mammals, the adverse cardiac remodelling, which compensates for the loss of cardiac cells, impairs cardiac function due to the non-contractile nature of fibrotic tissue. Moreover, the neovascularisation after MI is inadequate to restore blood flow to the infarcted myocardium. This review aims to synthesise the most recent insights into the molecular and cellular players involved in endogenous myocardial and vascular regeneration, facilitating the identification of mechanisms that could be targeted to trigger cardiac regeneration, reduce fibrosis, and improve functional recovery post-MI. Reprogramming adult cardiomyocytes to regain their proliferative potential, along with the modulation of target cells responsible for neovascularisation, represents promising therapeutic strategies. An updated overview of endogenous mechanisms that regulate both myocardial and coronary vasculature regeneration-including stem and progenitor cells, growth factors, cell cycle regulators, and key signalling pathways-could help identify new critical intervention points for therapeutic applications.
Keywords: Myocardial infarction; angiogenesis; cardiac regeneration; cardiomyocytes; endothelial cells; regenerative capacity; stem/progenitor cells; tissue repair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A collagen hydrogel loaded with HDAC7-derived peptide promotes the regeneration of infarcted myocardium with functional improvement in a rodent model.Acta Biomater. 2019 Mar 1;86:223-234. doi: 10.1016/j.actbio.2019.01.022. Epub 2019 Jan 16. Acta Biomater. 2019. PMID: 30660010
-
Expandable human cardiovascular progenitors from stem cells for regenerating mouse heart after myocardial infarction.Cardiovasc Res. 2020 Mar 1;116(3):545-553. doi: 10.1093/cvr/cvz181. Cardiovasc Res. 2020. PMID: 31287499 Free PMC article.
-
Multicellular Transcriptional Analysis of Mammalian Heart Regeneration.Circulation. 2017 Sep 19;136(12):1123-1139. doi: 10.1161/CIRCULATIONAHA.117.028252. Epub 2017 Jul 21. Circulation. 2017. PMID: 28733351 Free PMC article.
-
Angiogenesis after acute myocardial infarction.Cardiovasc Res. 2021 Apr 23;117(5):1257-1273. doi: 10.1093/cvr/cvaa287. Cardiovasc Res. 2021. PMID: 33063086 Review.
-
Adult Cardiomyocyte Proliferation: a New Insight for Myocardial Infarction Therapy.J Cardiovasc Transl Res. 2021 Jun;14(3):457-466. doi: 10.1007/s12265-020-10067-8. Epub 2020 Aug 20. J Cardiovasc Transl Res. 2021. PMID: 32820393 Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

