High-Affinity Plasma Membrane Ca2+ Channel Cch1 Modulates Adaptation to Sodium Dodecyl Sulfate-Triggered Rise in Cytosolic Ca2+ Concentration in Ogataea parapolymorpha
- PMID: 39519003
- PMCID: PMC11546840
- DOI: 10.3390/ijms252111450
High-Affinity Plasma Membrane Ca2+ Channel Cch1 Modulates Adaptation to Sodium Dodecyl Sulfate-Triggered Rise in Cytosolic Ca2+ Concentration in Ogataea parapolymorpha
Abstract
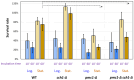
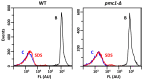
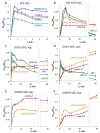
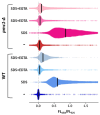
The cytosolic calcium concentration ([Ca2+]cyt) in yeast cells is maintained at a low level via the action of different transporters sequestrating these cations in the vacuole. Among them, the vacuolar Ca2+ ATPase Pmc1 crucially contributes to this process. Its inactivation in Ogataea yeasts was shown to cause sodium dodecyl sulfate (SDS) hypersensitivity that can be alleviated by the inactivation of the plasma membrane high-affinity Ca2+ channel Cch1. Here, we show that SDS at low concentrations induces a rapid influx of external Ca2+ into cells, while the plasma membrane remains impermeable for propidium iodide. The inactivation of Pmc1 disturbs efficient adaptation to this activity of SDS. The inactivation of Cch1 partially restores the ability of pmc1 mutant cells to cope with an increased [Ca2+]cyt that correlates with the suppression of SDS hypersensitivity. At the same time, Cch1 is unlikely to be directly involved in SDS-induced Ca2+ influx, since its inactivation does not decrease the amplitude of the rapid [Ca2+]cyt elevation in the pmc1-Δ mutant. The obtained data suggest that the effects of CCH1 inactivation on SDS sensitivity and coping with increased [Ca2+]cyt are related to an additional Cch1 function beyond its direct involvement in Ca2+ transport.
Keywords: Hog1 phosphorylation; Ogataea; genetically encoded Ca2+ indicator; high-affinity Ca2+ uptake system; vacuolar Ca2+ ATPase; voltage-gated calcium channel; yeast.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Inactivation of Pmc1 vacuolar Ca(2+) ATPase causes G(2) cell cycle delay in Hansenula polymorpha.Cell Cycle. 2012 Feb 15;11(4):778-84. doi: 10.4161/cc.11.4.19220. Cell Cycle. 2012. PMID: 22374675
-
The GEM-GECO Calcium Indicator Is Useable in Ogataea parapolymorpha Yeast, but Aggravates Effects of Increased Cytosolic Calcium Levels.Int J Mol Sci. 2022 Sep 2;23(17):10004. doi: 10.3390/ijms231710004. Int J Mol Sci. 2022. PMID: 36077401 Free PMC article.
-
Cch1 restores intracellular Ca2+ in fungal cells during endoplasmic reticulum stress.J Biol Chem. 2010 Apr 2;285(14):10951-8. doi: 10.1074/jbc.M109.056218. Epub 2010 Feb 1. J Biol Chem. 2010. PMID: 20123986 Free PMC article.
-
The plant vacuole: emitter and receiver of calcium signals.Cell Calcium. 2011 Aug;50(2):120-8. doi: 10.1016/j.ceca.2011.02.002. Epub 2011 Mar 4. Cell Calcium. 2011. PMID: 21376393 Review.
-
Genes for calcium-permeable channels in the plasma membrane of plant root cells.Biochim Biophys Acta. 2002 Aug 31;1564(2):299-309. doi: 10.1016/s0005-2736(02)00509-6. Biochim Biophys Acta. 2002. PMID: 12175911 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

