Design Principles and Applications of Fluorescent Kinase Inhibitors for Simultaneous Cancer Bioimaging and Therapy
- PMID: 39518106
- PMCID: PMC11545566
- DOI: 10.3390/cancers16213667
Design Principles and Applications of Fluorescent Kinase Inhibitors for Simultaneous Cancer Bioimaging and Therapy
Abstract
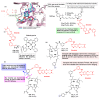
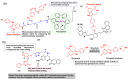
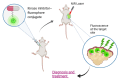
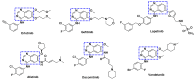
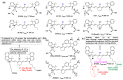
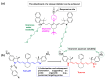
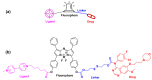
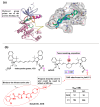
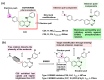
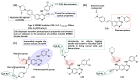
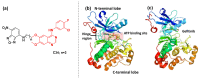
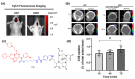
Kinase inhibitors are potent therapeutic agents in cancer treatment, but their effectiveness is frequently restricted by the inability to image the tumor microenvironment. To address this constraint, kinase inhibitor-fluorophore conjugates have emerged as promising theranostic agents, allowing for simultaneous cancer diagnosis and treatment. These conjugates are gaining attention for their ability to visualize malignant tissues and concurrently enhance therapeutic interventions. This review explores the design principles governing the development of multimodal inhibitors, highlighting their potential as platforms for kinase tracking and inhibition via bioimaging. The structural aspects of constructing such theranostic agents are critically analyzed. This work could shed light on this intriguing field and provide adequate impetus for developing novel theranostic compounds based on small molecule inhibitors and fluorophores.
Keywords: bioimaging; conjugates; design principles; kinase inhibitors; theranostic agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Theranostic nanoshells: from probe design to imaging and treatment of cancer.Acc Chem Res. 2011 Oct 18;44(10):936-46. doi: 10.1021/ar200023x. Epub 2011 May 25. Acc Chem Res. 2011. PMID: 21612199 Free PMC article. Review.
-
Multifunctional Theranostic Probe Based on the Pim-1 Kinase Inhibitor with the Function of Tracking pH Fluctuations during Treatment.Anal Chem. 2023 Aug 8;95(31):11732-11740. doi: 10.1021/acs.analchem.3c01818. Epub 2023 Jul 25. Anal Chem. 2023. PMID: 37490364
-
Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights.Acc Chem Res. 2018 Jun 19;51(6):1404-1414. doi: 10.1021/acs.accounts.8b00060. Epub 2018 May 7. Acc Chem Res. 2018. PMID: 29733571
-
Design Principles Governing the Development of Theranostic Anticancer Agents and Their Nanoformulations with Photoacoustic Properties.Pharmaceutics. 2022 Feb 4;14(2):362. doi: 10.3390/pharmaceutics14020362. Pharmaceutics. 2022. PMID: 35214094 Free PMC article. Review.
-
Engineering Persistent Luminescence Nanoparticles for Biological Applications: From Biosensing/Bioimaging to Theranostics.Acc Chem Res. 2018 May 15;51(5):1131-1143. doi: 10.1021/acs.accounts.7b00619. Epub 2018 Apr 17. Acc Chem Res. 2018. PMID: 29664602
References
-
- Cancer Statistics. (n.d.). Cancer.gov. [(accessed on 1 February 2023)]; Available online: https://www.cancer.gov/about-cancer/understanding/statistics.
-
- Gupta S., Howard S.C., Hunger S.P., Antillon F.G., Metzger M.L., Israels T., Harif M., Rodriguez-Galindo C. Treating Childhood Cancer in Low- and Middle-Income Countries. In: Gelband H., Jha P., Sankaranarayanan R., Horton S., editors. Cancer: Disease Control Priorities. 3rd ed. Volume 3 The International Bank for Reconstruction and Development/The World Bank© 2015 International Bank for Reconstruction and Development the World Bank; Washington, DC, USA: 2015. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

