The Crosstalk of Apoptotic and Non-Apoptotic Signaling in CD95 System
- PMID: 39513921
- PMCID: PMC11545656
- DOI: 10.3390/cells13211814
The Crosstalk of Apoptotic and Non-Apoptotic Signaling in CD95 System
Abstract
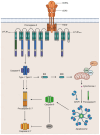
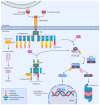
The mechanisms of CD95 (Fas/APO-1)-mediated extrinsic apoptotic pathway in cancer cells have been extensively studied. The majority of human cells express CD95, but not all these cells can induce extrinsic apoptosis. Accumulating evidence has shown that CD95 is a multifunctional protein, and its stimulation can also elicit non-apoptotic or even survival signals. It has become clear that under certain cellular contexts, due to the various checkpoints, CD95 activation can trigger both apoptotic and non-apoptotic signals. The crosstalk of death and survival signals may occur at different levels of signal transduction. The strength of the CD95 stimulation, initial levels of anti-apoptotic proteins, and posttranslational modifications of the core DISC components have been proposed to be the most important factors in the life/death decisions at CD95. Successful therapeutic targeting of CD95 signaling pathways will require a better understanding of the crosstalk between CD95-induced apoptotic and cell survival pathways. In this review, in order to gain a systematic understanding of the crosstalk between CD95-mediated apoptosis and non-apoptotic signaling, we will discuss these issues in a step-by-step way.
Keywords: CD95; DISC; caspase; extrinsic apoptosis; non-apoptotic signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulation of CD95/Fas signaling at the DISC.Cell Death Differ. 2012 Jan;19(1):36-41. doi: 10.1038/cdd.2011.155. Epub 2011 Nov 11. Cell Death Differ. 2012. PMID: 22075988 Free PMC article. Review.
-
Protein kinase C regulates FADD recruitment and death-inducing signaling complex formation in Fas/CD95-induced apoptosis.J Biol Chem. 2001 Nov 30;276(48):44944-52. doi: 10.1074/jbc.M104919200. Epub 2001 Oct 1. J Biol Chem. 2001. PMID: 11581255
-
Caspase-2 is activated at the CD95 death-inducing signaling complex in the course of CD95-induced apoptosis.Blood. 2006 Jul 15;108(2):559-65. doi: 10.1182/blood-2005-07-007096. Blood. 2006. PMID: 16822901
-
Modulation of CD95-mediated signaling by post-translational modifications: towards understanding CD95 signaling networks.Apoptosis. 2019 Jun;24(5-6):385-394. doi: 10.1007/s10495-019-01540-0. Apoptosis. 2019. PMID: 31069559 Review.
-
Caspase-8 tyrosine-380 phosphorylation inhibits CD95 DISC function by preventing procaspase-8 maturation and cycling within the complex.Oncogene. 2016 Oct 27;35(43):5629-5640. doi: 10.1038/onc.2016.99. Epub 2016 Apr 25. Oncogene. 2016. PMID: 27109099 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

