Targeted Delivery to Dying Cells Through P-Selectin-PSGL-1 Axis: A Promising Strategy for Enhanced Drug Efficacy in Liver Injury Models
- PMID: 39513885
- PMCID: PMC11545035
- DOI: 10.3390/cells13211778
Targeted Delivery to Dying Cells Through P-Selectin-PSGL-1 Axis: A Promising Strategy for Enhanced Drug Efficacy in Liver Injury Models
Abstract
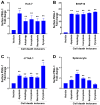
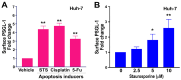
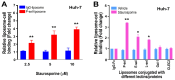
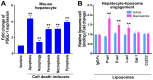
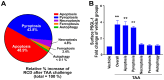
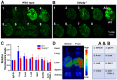
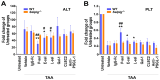
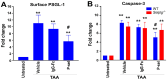
To minimize off-target adverse effects and improve drug efficacy, various tissue-specific drug delivery systems have been developed. However, even in diseased organs, both normal and stressed, dying cells coexist, and a targeted delivery system specifically for dying cells has yet to be explored to mitigate off-target effects within the same organ. This study aimed to establish such a system. By examining the surfaces of dying cells in vitro, we identified P-selectin glycoprotein ligand-1 (PSGL-1) as a universal marker for dying cells, positioning it as a potential target for selective drug delivery. We demonstrated that liposomes conjugated with the PSGL-1 binding protein P-selectin had significantly greater binding efficiency to dying cells compared to control proteins such as E-selectin, L-selectin, galectin-1, and C-type lectin-like receptor 2. Using thioacetamide (TAA) to induce hepatitis and hepatocyte damage in mice, we assessed the effectiveness of our P-selectin-based delivery system. In vivo, P-selectin-conjugated liposomes effectively delivered fluorescent dye and the apoptosis inhibitor z-DEVD to TAA-damaged livers in wild-type mice, but not in PSGL-1 knockout mice. In TAA-treated wild-type mice, unconjugated liposomes required a 100-fold higher z-DEVD dose compared to P-selectin-conjugated liposomes to achieve a comparable, albeit less effective, therapeutic outcome in lowering plasma alanine transaminase levels and alleviating thrombocytopenia. This emphasizes that P-selectin conjugation enhances drug delivery efficiency by approximately 100-fold in mice. These results suggest that P-selectin-based liposomes could be a promising strategy for targeted drug delivery, enabling both diagnosis and treatment by specifically delivering cell-labeling agents and rescue agents to dying cells via the P-selectin-PSGL-1 axis at the individual cell level.
Keywords: apoptosis inhibitor z-DEVD; drug-induced liver damage; dying-cell-specific drug delivery system; fluorescent dye delivery; image tracking and diagnosis; regulated cell death.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Erratum: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs.J Vis Exp. 2023 May 26;(195). doi: 10.3791/6561. J Vis Exp. 2023. PMID: 37235796
-
Ethics of Procuring and Using Organs or Tissue from Infants and Newborns for Transplantation, Research, or Commercial Purposes: Protocol for a Bioethics Scoping Review.Wellcome Open Res. 2024 Dec 5;9:717. doi: 10.12688/wellcomeopenres.23235.1. eCollection 2024. Wellcome Open Res. 2024. PMID: 39839977 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Gadolinium Magnetic Resonance Imaging.2023 Jul 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Jul 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 29494094 Free Books & Documents.
-
Strategies to improve smoking cessation rates in primary care.Cochrane Database Syst Rev. 2021 Sep 6;9(9):CD011556. doi: 10.1002/14651858.CD011556.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693994 Free PMC article. Review.
References
-
- Zhang Y., Almazi J.G., Ong H.X., Johansen M.D., Ledger S., Traini D., Hansbro P.M., Kelleher A.D., Ahlenstiel C.L. Nanoparticle Delivery Platforms for RNAi Therapeutics Targeting COVID-19 Disease in the Respiratory Tract. Int. J. Mol. Sci. 2022;23:2408. doi: 10.3390/ijms23052408. - DOI - PMC - PubMed
-
- Markowski A., Jaromin A., Migdal P., Olczak E., Zygmunt A., Zaremba-Czogalla M., Pawlik K., Gubernator J. Design and Development of a New Type of Hybrid PLGA/Lipid Nanoparticle as an Ursolic Acid Delivery System against Pancreatic Ductal Adenocarcinoma Cells. Int. J. Mol. Sci. 2022;23:5536. doi: 10.3390/ijms23105536. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

