Impact of cytopenias and early versus late treatment with ruxolitinib in patients with steroid-refractory acute or chronic graft-versus-host disease
- PMID: 39506073
- PMCID: PMC11726446
- DOI: 10.1038/s41409-024-02445-6
Impact of cytopenias and early versus late treatment with ruxolitinib in patients with steroid-refractory acute or chronic graft-versus-host disease
Abstract
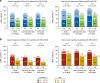
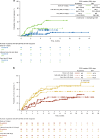
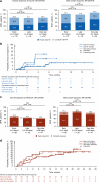
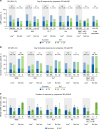
REACH2 and REACH3 were randomized, multicenter, open-label phase 3 studies comparing the selective Janus kinase (JAK)1/JAK2 inhibitor ruxolitinib versus investigators' choice of best available therapy (BAT) in steroid-refractory (SR) acute (REACH2) or chronic (REACH3) graft-versus-host disease (aGVHD/cGVHD). Moderate-severe aGVHD/cGVHD can progress rapidly; thus, key clinical considerations driving management of patients with SR-aGVHD/SR-cGVHD are prompt treatment initiation and concomitant cytopenias. These post hoc analyses of REACH2/REACH3 describe the impact of timing of treatment initiation after SR-aGVHD/SR-cGVHD diagnosis and development of concomitant cytopenias on treatment outcomes. Ruxolitinib initiation within 3 days from SR-aGVHD diagnosis yielded an extended duration of response and higher Day 28 complete response rates compared with initiation ≥7 days after SR-aGVHD diagnosis (median 178 vs 167 days and 36.6% vs 25.0%, respectively). For patients with SR-cGVHD, Week 24 overall response was not impacted by time to treatment (54.5% vs 42.6% for <14 vs >28 days). Clinically relevant cytopenias were manageable, allowing for maintenance of dose intensity (median 20 mg/d), and did not impact the favorable efficacy outcomes from ruxolitinib treatment. This analysis highlights the practical importance of considering earlier ruxolitinib initiation after SR diagnosis in GVHD and the benefits of ruxolitinib treatment compared with BAT even for patients with cytopenias.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: ZM: Advisory board participation: AstraZeneca, BMS, Genentech, Janssen, KITE, Pfizer. RZ: Consulting fees and speaker bureau: Incyte, Medac, Mallinckrodt Pharmaceuticals/Therakos, Neovii, Novartis, Sanofi. FL: Consulting fees and speaker bureau: Amgen, Gilead, Miltenyi, Novartis, Sanofi, SOBI. GS: Consulting fees and speaker bureau: Allovir, Incyte, Novartis, Sanofi. MM: Declared no competing interests. VB, JG, and ZX are employees and shareholders of Incyte. Ethical approval and consent to participate: Based on the retrospective post hoc analysis design of this study, no additional ethical approval or consent to participate was required. However, the REACH trials on which this study were based were designed and conducted in accordance with the International Council for Harmonisation Guideline for Good Clinical Practice, Declaration of Helsinki, and local regulatory requirements. Study protocols were approved by institutional review boards at each site. Patients or their guardians provided written informed consent for participation in the original REACH trials.
Figures
Similar articles
-
Ruxolitinib for steroid-refractory chronic graft-versus-host disease: Japanese subgroup analysis of REACH3 study.Int J Hematol. 2024 Dec;120(6):705-716. doi: 10.1007/s12185-024-03850-9. Epub 2024 Oct 3. Int J Hematol. 2024. PMID: 39361234 Free PMC article. Clinical Trial.
-
Ruxolitinib in patients with graft versus host disease (GvHD): findings from a compassionate use program.Bone Marrow Transplant. 2024 May;59(5):637-646. doi: 10.1038/s41409-024-02207-4. Epub 2024 Feb 15. Bone Marrow Transplant. 2024. PMID: 38361117 Free PMC article.
-
Efficacy and safety of human umbilical cord-derived mesenchymal stem cells versus placebo added to second-line therapy in patients with steroid-refractory acute graft-versus-host disease: a multicentre, randomized, double-blind, phase 2 trial.BMC Med. 2024 Nov 25;22(1):555. doi: 10.1186/s12916-024-03782-5. BMC Med. 2024. PMID: 39587570 Free PMC article. Clinical Trial.
-
Efficacy of Ruxolitinib with corticosteroids in idiopathic pneumonia syndrome post-allogeneic hematopoietic stem cell transplantation: A single-center experience and systematic review.Transpl Immunol. 2024 Dec;87:102135. doi: 10.1016/j.trim.2024.102135. Epub 2024 Oct 4. Transpl Immunol. 2024. PMID: 39368752
-
Oral budesonide for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2015 Oct 26;2015(10):CD007698. doi: 10.1002/14651858.CD007698.pub3. Cochrane Database Syst Rev. 2015. PMID: 26497719 Free PMC article. Review.
References
-
- Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385:228–38. - PubMed
-
- Jagasia M, Zeiser R, Arbushites M, Delaite P, Gadbaw B, Bubnoff NV. Ruxolitinib for the treatment of patients with steroid-refractory GVHD: an introduction to the REACH trials. Immunotherapy. 2018;10:391–402. - PubMed
-
- Zeiser R, Teshima T. Nonclassical manifestations of acute GVHD. Blood. 2021;138:2165–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

