Zeolitic Imidazolate Framework-8 Offers an Anti-Inflammatory and Antifungal Method in the Treatment of Aspergillus Fungal Keratitis in vitro and in vivo
- PMID: 39502641
- PMCID: PMC11537184
- DOI: 10.2147/IJN.S480800
Zeolitic Imidazolate Framework-8 Offers an Anti-Inflammatory and Antifungal Method in the Treatment of Aspergillus Fungal Keratitis in vitro and in vivo
Abstract
Background: Fungal keratitis is a serious blinding eye disease. Traditional drugs used to treat fungal keratitis commonly have the disadvantages of low bioavailability, poor dispersion, and limited permeability.
Purpose: To develop a new method for the treatment of fungal keratitis with improved bioavailability, dispersion, and permeability.
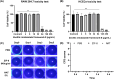
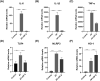
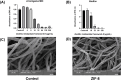
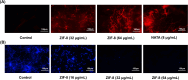
Methods: Zeolitic Imidazolate Framework-8 (ZIF-8) was formed by zinc ions and 2-methylimidazole linked by coordination bonds and characterized by Scanning electron microscopy (SEM), X-ray diffraction (XRD), and Zeta potential. The safety of ZIF-8 on HCECs and RAW 264.7 cells was detected by Cell Counting Kit-8 (CCK-8). Safety evaluation of ZIF-8 on mice corneal epithelium was conducted using the Draize corneal toxicity test. The effects of ZIF-8 on fungal growth, biofilm formation, and hyphae structure were detected by Minimal inhibit concentration (MIC), crystal violet staining, Propidium Iodide (PI) testing, and calcofluor white staining. The anti-inflammatory effects of ZIF-8 on RAW 246.7 cells were evaluated by Quantitative Real-Time PCR Experiments (qPCR) and Enzyme-linked immunosorbent assay (ELISA). Clinical score, Colony-Forming Units (CFU), Hematoxylin-eosin (HE) staining, and immunofluorescence were conducted to verify the therapeutic effect of ZIF-8 on C57BL/6 female mice with fungal keratitis.
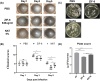
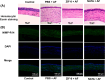
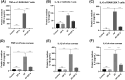
Results: In vitro, ZIF-8 showed outstanding antifungal effects, including inhibiting the growth of Aspergillus fumigatus over 90% at 64 μg/mL, restraining the formation of biofilm, and destroying cell membranes. In vivo, treatment with ZIF-8 reduced corneal fungal load and mitigated neutrophil infiltration in fungal keratitis, which effectively reduced the severity of keratitis in mice and alleviated the infiltration of inflammatory factors in the mouse cornea. In addition, ZIF-8 reduces the inflammatory response by downregulating the expression of pro-inflammatory cytokines TNF-α, IL-6, and IL-1β after Aspergillus fumigatus infection in vivo and in vitro.
Conclusion: ZIF-8 has a significant anti-inflammatory and antifungal effect, which provides a new solution for the treatment of fungal keratitis.
Keywords: MOFs; ZIF-8; anti-inflammatory; antifungal; fungal keratitis.
© 2024 Fu et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Baicalein Protects Against Aspergillus fumigatus Keratitis by Reducing Fungal Load and Inhibiting TSLP-Induced Inflammatory Response.Invest Ophthalmol Vis Sci. 2021 May 3;62(6):26. doi: 10.1167/iovs.62.6.26. Invest Ophthalmol Vis Sci. 2021. PMID: 34038512 Free PMC article.
-
Isorhamnetin Ameliorates Aspergillus fumigatus Keratitis by Reducing Fungal Load, Inhibiting Pattern-Recognition Receptors and Inflammatory Cytokines.Invest Ophthalmol Vis Sci. 2021 Mar 1;62(3):38. doi: 10.1167/iovs.62.3.38. Invest Ophthalmol Vis Sci. 2021. PMID: 33783487 Free PMC article.
-
Gallic Acid Ameliorates Aspergillus Fumigatus Keratitis Through Reducing Fungal Load and Suppressing the Inflammatory Response.Invest Ophthalmol Vis Sci. 2022 Nov 1;63(12):12. doi: 10.1167/iovs.63.12.12. Invest Ophthalmol Vis Sci. 2022. PMID: 36350620 Free PMC article.
-
The role of Glabridin in antifungal and anti-inflammation effects in Aspergillus fumigatus keratitis.Exp Eye Res. 2022 Jan;214:108883. doi: 10.1016/j.exer.2021.108883. Epub 2021 Dec 8. Exp Eye Res. 2022. PMID: 34896107
-
Formononetin protects against Aspergillus fumigatus Keratitis: Targeting inflammation and fungal load.Int Immunopharmacol. 2024 May 10;132:112046. doi: 10.1016/j.intimp.2024.112046. Epub 2024 Apr 9. Int Immunopharmacol. 2024. PMID: 38593508
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

