Macroporous chitosan/alginate hydrogels crosslinked with genipin accumulate and retain glioblastoma cancer cells
- PMID: 39502176
- PMCID: PMC11537210
- DOI: 10.1039/d4ra06197g
Macroporous chitosan/alginate hydrogels crosslinked with genipin accumulate and retain glioblastoma cancer cells
Abstract
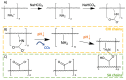
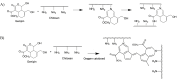
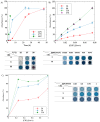
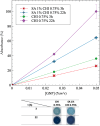
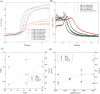
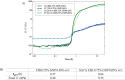
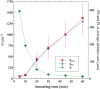
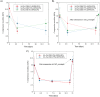
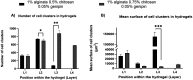
Grade IV multiforme glioblastoma (GBM) is an aggressive cancer that remains incurable due to the GBM cells invading and proliferating in the surrounding healthy tissues, even after tumor resection. A new therapeutic paradigm to treat GBM is to attract and accumulate GBM cells in a macroporous hydrogel inserted in the surgical cavity after tumor resection, followed by a targeted high dose of radiotherapy. This work presents a molding-based method to prepare macroporous hydrogels composed of sodium alginate and chitosan, homogeneously mixed in solution using sodium bicarbonate, and subsequently crosslinked with genipin and calcium chloride. The gels display a blue color, the result of chitosan crosslinking with genipin, fully interconnected pores with an average diameter of 180 μm (and tunable over a wide range), with a compression modulus of 10 kPa, close to the value of brain tissues. The gels are stable in cell culture media and keep their integrity after radiation doses comparable to current GBM treatment levels. Finally, F98 GBM cells accumulate relatively homogeneously and are retained within the gels.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
The role of pore size and mechanical properties on the accumulation, retention and distribution of F98 glioblastoma cells in macroporous hydrogels.Biomed Mater. 2024 Jun 26;19(4). doi: 10.1088/1748-605X/ad581b. Biomed Mater. 2024. PMID: 38870993
-
Effect of Chitosan on Alginate-Based Macroporous Hydrogels for the Capture of Glioblastoma Cancer Cells.ACS Appl Bio Mater. 2022 Aug 10. doi: 10.1021/acsabm.2c00598. Online ahead of print. ACS Appl Bio Mater. 2022. PMID: 35948423
-
An alginate-based macroporous hydrogel matrix to trap cancer cells.Carbohydr Polym. 2021 Aug 15;266:118115. doi: 10.1016/j.carbpol.2021.118115. Epub 2021 Apr 24. Carbohydr Polym. 2021. PMID: 34044932
-
Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone.Mar Drugs. 2015 Dec 11;13(12):7314-38. doi: 10.3390/md13127068. Mar Drugs. 2015. PMID: 26690453 Free PMC article. Review.
-
Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma.J Control Release. 2016 Dec 10;243:29-42. doi: 10.1016/j.jconrel.2016.09.034. Epub 2016 Sep 28. J Control Release. 2016. PMID: 27693428 Review.
References
-
- D'Alessandris Q. G. Biffoni M. Martini M. Runci D. Buccarelli M. Cenci T. Signore M. Stancato L. Olivi A. De Maria R. Larocca L. M. Ricci-Vitiani L. Pallini R. The clinical value of patient-derived glioblastoma tumorspheres in predicting treatment response. Neuro-Oncology. 2017;19:1097–1108. - PMC - PubMed
LinkOut - more resources
Full Text Sources

