Rab2A-mediated Golgi-lipid droplet interactions support very-low-density lipoprotein secretion in hepatocytes
- PMID: 39496977
- PMCID: PMC11649929
- DOI: 10.1038/s44318-024-00288-x
Rab2A-mediated Golgi-lipid droplet interactions support very-low-density lipoprotein secretion in hepatocytes
Abstract
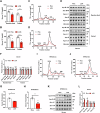
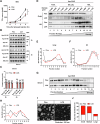
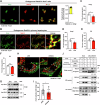
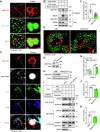
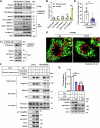
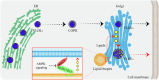
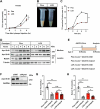
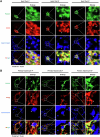
Lipid droplets (LDs) serve as crucial hubs for lipid trafficking and metabolic regulation through their numerous interactions with various organelles. While the interplay between LDs and the Golgi apparatus has been recognized, their roles and underlying mechanisms remain poorly understood. Here, we reveal the role of Ras-related protein Rab-2A (Rab2A) in mediating LD-Golgi interactions, thereby contributing to very-low-density lipoprotein (VLDL) lipidation and secretion in hepatocytes. Mechanistically, our findings identify a selective interaction between Golgi-localized Rab2A and 17-beta-hydroxysteroid dehydrogenase 13 (HSD17B13) protein residing on LDs. This complex facilitates dynamic organelle communication between the Golgi apparatus and LDs, thus contributing to lipid transfer from LDs to the Golgi apparatus for VLDL2 lipidation and secretion. Attenuation of Rab2A activity via AMP-activated protein kinase (AMPK) suppresses the Rab2A-HSD17B13 complex formation, impairing LD-Golgi interactions and subsequent VLDL secretion. Furthermore, genetic inhibition of Rab2A and HSD17B13 in the liver reduces the serum triglyceride and cholesterol levels. Collectively, this study provides a new perspective on the interactions between the Golgi apparatus and LDs.
Keywords: 17-Beta-hydroxysteroid Dehydrogenase 13; AMP-activated Protein Kinase; Organelle Interactions; Ras-related Protein Rab-2A; Very-low-density Lipoprotein.
© 2024. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures
Similar articles
-
Fisetin Ameliorates Hepatocyte Lipid Droplet Accumulation via Targeting the Rhythmic Protein BMAL1 to Regulate Cell Death-Inducing DNA Fragmentation Factor-α-like Effector C-Mediated Lipid Droplet Fusion.J Agric Food Chem. 2024 Dec 4;72(48):26884-26897. doi: 10.1021/acs.jafc.4c06487. Epub 2024 Nov 20. J Agric Food Chem. 2024. PMID: 39563624
-
Female-age-dependent changes in the lipid fingerprint of the mammalian oocytes.Hum Reprod. 2024 Dec 1;39(12):2754-2767. doi: 10.1093/humrep/deae225. Hum Reprod. 2024. PMID: 39366679 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
FITM2 deficiency results in ER lipid accumulation, ER stress, and reduced apolipoprotein B lipidation and VLDL triglyceride secretion in vitro and in mouse liver.Mol Metab. 2024 Dec;90:102048. doi: 10.1016/j.molmet.2024.102048. Epub 2024 Oct 18. Mol Metab. 2024. PMID: 39426520 Free PMC article.
-
Mobile phone text messaging for medication adherence in secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2024 Mar 27;3(3):CD011851. doi: 10.1002/14651858.CD011851.pub3. Cochrane Database Syst Rev. 2024. PMID: 38533994 Free PMC article. Review.
References
-
- Adam M, Heikelä H, Sobolewski C, Portius D, Mäki-Jouppila J, Mehmood A, Adhikari P, Esposito I, Elo LL, Zhang F-P et al (2018) Hydroxysteroid (17β) dehydrogenase 13 deficiency triggers hepatic steatosis and inflammation in mice. FASEB J 32:3434–3447 - PubMed
-
- Barbosa AD, Savage DB, Siniossoglou S (2015) Lipid droplet-organelle interactions: emerging roles in lipid metabolism. Curr Opin Cell Biol 35:91–97 - PubMed
MeSH terms
Substances
Grants and funding
- 82000549/MOST | National Natural Science Foundation of China (NSFC)
- 32025019/MOST | National Natural Science Foundation of China (NSFC)
- 82470614/MOST | National Natural Science Foundation of China (NSFC)
- 2008085MC67/| Natural Science Foundation of Anhui Province ()
- 0810013101/Doctoral Start-up Foundation of Anhui Medical University
LinkOut - more resources
Full Text Sources
Research Materials

