Multiscale, mechanistic model of Rheumatoid Arthritis to enable decision making in late stage drug development
- PMID: 39496637
- PMCID: PMC11535547
- DOI: 10.1038/s41540-024-00454-1
Multiscale, mechanistic model of Rheumatoid Arthritis to enable decision making in late stage drug development
Abstract
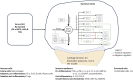
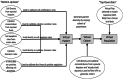
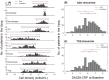
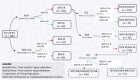
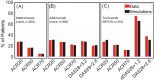
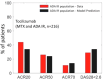
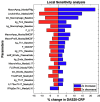
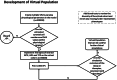
Rheumatoid Arthritis (RA) is a chronic autoimmune inflammatory disease that affects about 0.1% to 2% of the population worldwide. Despite the development of several novel therapies, there is only limited benefit for many patients. Thus, there is room for new approaches to improve response to therapy, including designing better trials e.g., by identifying subpopulations that can benefit from specific classes of therapy and enabling reverse translation by analyzing completed clinical trials. We have developed an open-source, mechanistic multi-scale model of RA, which captures the interactions of key immune cells and mediators in an inflamed joint. The model consists of a treatment-naive Virtual Population (Vpop) that responds appropriately (i.e. as reported in clinical trials) to standard-of-care treatment options-Methotrexate (MTX) and Adalimumab (ADA, anti-TNF-α) and an MTX inadequate responder sub-population that responds appropriately to Tocilizumab (TCZ, anti-IL-6R) therapy. The clinical read-outs of interest are the American College of Rheumatology score (ACR score) and Disease Activity Score (DAS28-CRP), which is modeled to be dependent on the physiological variables in the model. Further, we have validated the Vpop by predicting the therapy response of TCZ on ADA Non-responders. This paper aims to share our approach, equations, and code to enable community evaluation and greater adoption of mechanistic models in drug development for autoimmune diseases.
© 2024. The Author(s).
Conflict of interest statement
DB, RM, MC, TR, PP and RK all declare that they have no competing financial or non-financial interests.
Figures

Similar articles
-
Effect of Drug Therapy on Net Cholesterol Efflux Capacity of High-Density Lipoprotein-Enriched Serum in Rheumatoid Arthritis.Arthritis Rheumatol. 2016 Sep;68(9):2099-105. doi: 10.1002/art.39675. Arthritis Rheumatol. 2016. PMID: 26991245 Free PMC article.
-
Clinical, functional, and radiographic implications of time to treatment response in patients with early rheumatoid arthritis: a posthoc analysis of the PREMIER study.J Rheumatol. 2014 Feb;41(2):235-43. doi: 10.3899/jrheum.121468. Epub 2013 Dec 1. J Rheumatol. 2014. PMID: 24293583 Clinical Trial.
-
Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation.Health Technol Assess. 2016 Apr;20(35):1-610. doi: 10.3310/hta20350. Health Technol Assess. 2016. PMID: 27140438 Free PMC article. Review.
-
Comparison of the effects of tocilizumab monotherapy and adalimumab in combination with methotrexate on bone erosion repair in rheumatoid arthritis.Ann Rheum Dis. 2019 Sep;78(9):1186-1191. doi: 10.1136/annrheumdis-2018-214894. Epub 2019 May 29. Ann Rheum Dis. 2019. PMID: 31142474
-
Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease-modifying anti-rheumatic drugs: a systematic review and network meta-analysis.Cochrane Database Syst Rev. 2016 May 13;2016(5):CD012183. doi: 10.1002/14651858.CD012183. Cochrane Database Syst Rev. 2016. PMID: 27175934 Free PMC article. Review.
References
-
- Almutairi, K. A.-O. et al. The Prevalence of Rheumatoid Arthritis: A Systematic Review of Population-based Studies. J. Rheumatol.48, 669–676 (2021). (0315-162X (Print)). - PubMed
-
- Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim.4, 18001 (2018). - PubMed
-
- van Riel, P. L., The development of the disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28). Clin Exp Rheumatol, 32: p. S-65–74. 2014) - PubMed
-
- Kay, J. & Upchurch, K. S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology51, vi5–vi9 (2012). - PubMed
-
- Singh, J. A. et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken)68, 1–25 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

