Efficacy of rechallenge after first-line immunotherapy for advanced gastric cancer: A retrospective real-world study
- PMID: 39494935
- PMCID: PMC11540071
- DOI: 10.1080/21645515.2024.2423479
Efficacy of rechallenge after first-line immunotherapy for advanced gastric cancer: A retrospective real-world study
Abstract
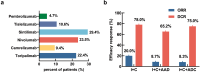
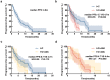
We aimed to explore the efficacy of rechallenge after first-line immunotherapy in advanced gastric cancer (AGC) and to analyze the factors affecting prognosis based on clinical characteristics. Eighty-five AGC patients who underwent rechallenged after the failure of first-line treatment with immune checkpoint inhibitors (ICIs) were retrospectively collected from July 2019 to December 2022 in Jiangsu Cancer Hospital. Potential factors affecting prognosis were analyzed by univariate and multivariate Cox analysis. Survival analysis was performed by Kaplan-Meier method and Log rank test. Stratified factors included human epidermal growth factor receptor 2 (HER-2) and programmed cell death-ligand 1 combined positive score (PD-L1 CPS). The objective response rate (ORR) was 15.3%, and the disease control rate (DCR) was 74.1%. The median progression-free survival (PFS) was 4.8 months. Results showed that patients in the I + C group had the best response. The ORR was 20.0% VS 8.7% in the I + C group and I + C + AAD group. The DCR was 78.0% VS 65.2%, and the median PFS was 6.7 VS 4.7 months [hazard ratio (HR): 0.55, 95% confidence interval (CI): 0.30-1.00, p = .022]. The ORR was 20.0% VS 8.3% in the I + C group and I + C + ADC group. The DCR was 78.0% VS 75.0%, and the median PFS was 6.7 VS 4.4 months (HR: 0.59, 95%CI: 0.26-1.30, p = .112). The median PFS was 4.7 VS 4.4 months in the I + C + AAD group and I + C + ADC group (HR: 1.21, 95%CI: 0.60-2.47, p = .580). Adverse events (AEs) were found in 34 patients, mainly including leukopenia 9 (10.6%), and neutropenia 8 (9.4%). The incidence of grade 3-4 AEs was 8.2%. There were no drug-related deaths and all AEs were manageable. Rechallenge after first-line immunotherapy showed good survival benefit and acceptable safety in the therapy of AGC. Especially for patients with HER-2-positive and PD-L1 CPS ≥ 1%, rechallenge may be an effective treatment modality.
Keywords: Advanced gastric cancer; efficacy; immune checkpoint inhibitors; rechallenge; safety.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Aug 27;8:CD007807. doi: 10.1002/14651858.CD007807.pub4 PMID: 28752910 Free PMC article. Updated. Review.
-
Interventions for supporting pregnant women's decision-making about mode of birth after a caesarean.Cochrane Database Syst Rev. 2013 Jul 30;2013(7):CD010041. doi: 10.1002/14651858.CD010041.pub2. Cochrane Database Syst Rev. 2013. PMID: 23897547 Free PMC article. Review.
References
-
- Masetti M, Al-Batran SE, Goetze TO, Thuss-Patience P, Knorrenschild JR, Goekkurt E, Folprecht G, Ettrich TJ, Lindig U, Luley KB, et al. Efficacy of ramucirumab combination chemotherapy as second-line treatment in patients with advanced adenocarcinoma of the stomach or gastroesophageal junction after exposure to checkpoint inhibitors and chemotherapy as first-line therapy. Intl J Cancer. 2024. June 15;154(12):2142–2150. doi:10.1002/ijc.34894. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
