Xianling Lianxia formula improves the efficacy of trastuzumab by enhancing NK cell-mediated ADCC in HER2-positive BC
- PMID: 39493309
- PMCID: PMC11531627
- DOI: 10.1016/j.jpha.2024.100977
Xianling Lianxia formula improves the efficacy of trastuzumab by enhancing NK cell-mediated ADCC in HER2-positive BC
Abstract
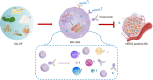
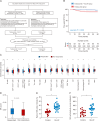
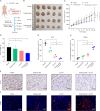
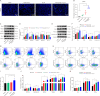
Trastuzumab has improved survival rates in human epidermal growth factor receptor 2 (HER2)-positive breast cancer (BC), but drug resistance leads to treatment failure. Natural killer (NK) cell-mediated antibody-dependent cell cytotoxicity (ADCC) represents an essential antitumor immune mechanism of trastuzumab. Traditional Chinese medicine (TCM) has been used for centuries to treat diseases because of its capacity to improve immune responses. Xianling Lianxia formula (XLLXF), based on the principle of "strengthening body and eliminating toxin", exhibits a synergistic effect in the trastuzumab treatment of patients with HER2-positive BC. Notably, this synergistic effect of XLLXF was executed by enhancing NK cells and ADCC, as demonstrated through in vitro co-culture of NK cells and BC cells and in vivo intervention experiments. Mechanistically, the augmented impact of XLLXF on NK cells is linked to a decrease in cytokine inducible Src homology 2 (SH2) containing protein (CISH) expression, which in turn activates the Janus kinase 1 (JAK1)/signal transducer and activator of transcription 5 (STAT5) pathway. Collectively, these findings suggested that XLLXF holds promise for enhancing NK cell function and sensitizing patients with HER2-positive BC to trastuzumab.
Keywords: ADCC effect; HER2-positive breast cancer; NK cell; Traditional chinese medicine; Trastuzumab.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



Similar articles
-
Xianling Lianxia formula enhances the inhibitory effects of trastuzumab on HER2-positive breast cancer.Acta Biochim Biophys Sin (Shanghai). 2024 Mar 25;56(3):462-473. doi: 10.3724/abbs.2023281. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38379418 Free PMC article.
-
Taxanes enhance trastuzumab-mediated ADCC on tumor cells through NKG2D-mediated NK cell recognition.Oncotarget. 2016 Jan 5;7(1):255-65. doi: 10.18632/oncotarget.6353. Oncotarget. 2016. PMID: 26595802 Free PMC article.
-
KIR-HLA Functional Repertoire Influences Trastuzumab Efficiency in Patients With HER2-Positive Breast Cancer.Front Immunol. 2022 Jan 12;12:791958. doi: 10.3389/fimmu.2021.791958. eCollection 2021. Front Immunol. 2022. PMID: 35095867 Free PMC article. Clinical Trial.
-
Focusing on NK cells and ADCC: A promising immunotherapy approach in targeted therapy for HER2-positive breast cancer.Front Immunol. 2022 Dec 19;13:1083462. doi: 10.3389/fimmu.2022.1083462. eCollection 2022. Front Immunol. 2022. PMID: 36601109 Free PMC article. Review.
-
Targeting ADCC: A different approach to HER2 breast cancer in the immunotherapy era.Breast. 2021 Dec;60:15-25. doi: 10.1016/j.breast.2021.08.007. Epub 2021 Aug 19. Breast. 2021. PMID: 34454323 Free PMC article. Review.
References
-
- Siegel R.L., Miller K.D., Wagle N.S., et al. Cancer statistics, 2023. CA Cancer J. Clin. 2023;73:17–48. - PubMed
-
- Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. - PubMed
-
- Slamon D.J., Leyland-Jones B., Shak S., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

