Heterogeneous Slowdown of Dynamics in the Condensate of an Intrinsically Disordered Protein
- PMID: 39486437
- PMCID: PMC11571228
- DOI: 10.1021/acs.jpclett.4c02142
Heterogeneous Slowdown of Dynamics in the Condensate of an Intrinsically Disordered Protein
Abstract
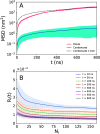
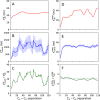
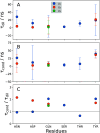
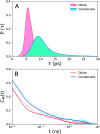
The high concentration of proteins and other biological macromolecules inside biomolecular condensates leads to dense and confined environments, which can affect the dynamic ensembles and the time scales of the conformational transitions. Here, we use atomistic molecular dynamics (MD) simulations of the intrinsically disordered low complexity domain (LCD) of the human fused in sarcoma (FUS) RNA-binding protein to study how self-crowding inside a condensate affects the dynamic motions of the protein. We found a heterogeneous retardation of the protein dynamics in the condensate with respect to the dilute phase, with large-amplitude motions being strongly slowed by up to 2 orders of magnitude, whereas small-scale motions, such as local backbone fluctuations and side-chain rotations, are less affected. The results support the notion of a liquid-like character of the condensates and show that different protein motions respond differently to the environment.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Conformational Dynamics of Intrinsically Disordered Proteins Regulate Biomolecular Condensate Chemistry.Chem Rev. 2022 Mar 23;122(6):6719-6748. doi: 10.1021/acs.chemrev.1c00774. Epub 2022 Feb 18. Chem Rev. 2022. PMID: 35179885 Free PMC article. Review.
-
Reversible Kinetic Trapping of FUS Biomolecular Condensates.Adv Sci (Weinh). 2022 Feb;9(4):e2104247. doi: 10.1002/advs.202104247. Epub 2021 Dec 4. Adv Sci (Weinh). 2022. PMID: 34862761 Free PMC article.
-
Simulation of FUS Protein Condensates with an Adapted Coarse-Grained Model.J Chem Theory Comput. 2021 Jan 12;17(1):525-537. doi: 10.1021/acs.jctc.0c01064. Epub 2020 Dec 13. J Chem Theory Comput. 2021. PMID: 33307683 Free PMC article.
-
Conformational Freedom and Topological Confinement of Proteins in Biomolecular Condensates.J Mol Biol. 2022 Jan 15;434(1):167348. doi: 10.1016/j.jmb.2021.167348. Epub 2021 Nov 9. J Mol Biol. 2022. PMID: 34767801 Free PMC article. Review.
-
Thermodynamic forces from protein and water govern condensate formation of an intrinsically disordered protein domain.Nat Commun. 2023 Sep 21;14(1):5892. doi: 10.1038/s41467-023-41586-y. Nat Commun. 2023. PMID: 37735186 Free PMC article.
Cited by
-
Mesoscale properties of biomolecular condensates emerging from protein chain dynamics.ArXiv [Preprint]. 2024 Jul 27:arXiv:2407.19202v1. ArXiv. 2024. PMID: 39398199 Free PMC article. Preprint.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

