Unlocking the regenerative key: Targeting stem cell factors for bone renewal
- PMID: 39479284
- PMCID: PMC11523181
- DOI: 10.1177/20417314241287491
Unlocking the regenerative key: Targeting stem cell factors for bone renewal
Abstract
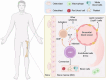
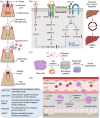
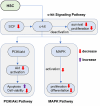
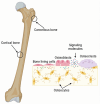
Stem cell factors (SCFs) are pivotal factors existing in both soluble and membrane-bound forms, expressed by endothelial cells (ECs) and fibroblasts throughout the body. These factors enhance cell growth, viability, and migration in multipotent cell lineages. The preferential expression of SCF by arteriolar ECs indicates that arterioles create a unique microenvironment tailored to hematopoietic stem cells (HSCs). Insufficiency of SCF within bone marrow (BM)-derived adipose tissue results in decreased their overall cellularity, affecting HSCs and their immediate progenitors critical for generating diverse blood cells and maintaining the hematopoietic microenvironment. SCF deficiency disrupts BM function, impacting the production and differentiation of HSCs. Additionally, deleting SCF from adipocytes reduces lipogenesis, highlighting the crucial role of SCF/c-kit signaling in controlling lipid accumulation. This review elucidates the sources, roles, mechanisms, and molecular strategies of SCF in bone renewal, offering a comprehensive overview of recent advancements, challenges, and future directions for leveraging SCF as a key agent in regenerative medicine.
Keywords: Stem cell factors; bone renewal; ligands; medicine; regeneration; viability.
© The Author(s) 2024.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
The membrane-bound isoform of stem cell factor synergizes with soluble flt3 ligand in supporting early hematopoietic cells in long-term cultures of normal and aplastic anemia bone marrow.Exp Hematol. 1998 May;26(5):365-73. Exp Hematol. 1998. PMID: 9590652
-
Murine hematopoietic stem cells and multipotent progenitors express truncated intracellular form of c-kit receptor.Stem Cells Dev. 2008 Apr;17(2):343-53. doi: 10.1089/scd.2007.0101. Stem Cells Dev. 2008. PMID: 18447649 Free PMC article.
-
Stem cell factor deficiency is vasculoprotective: unraveling a new therapeutic potential of imatinib mesylate.Circ Res. 2006 Sep 15;99(6):617-25. doi: 10.1161/01.RES.0000243210.79654.fd. Epub 2006 Aug 24. Circ Res. 2006. PMID: 16931795
-
Rebuilding the hematopoietic stem cell niche: Recent developments and future prospects.Acta Biomater. 2021 Sep 15;132:129-148. doi: 10.1016/j.actbio.2021.03.061. Epub 2021 Apr 1. Acta Biomater. 2021. PMID: 33813090 Review.
-
Bone marrow adiposity and the hematopoietic niche: A historical perspective of reciprocity, heterogeneity, and lineage commitment.Best Pract Res Clin Endocrinol Metab. 2021 Jul;35(4):101564. doi: 10.1016/j.beem.2021.101564. Epub 2021 Aug 10. Best Pract Res Clin Endocrinol Metab. 2021. PMID: 34417114 Review.
References
-
- Broudy VC. Stem Cell Factor and hematopoiesis. Blood 1997; 90(4): 1345–1364. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

