Cell aggregation mediated by ACE2 deletion in Candida auris modulates fungal colonization and host immune responses in the skin
- PMID: 39475280
- PMCID: PMC11580408
- DOI: 10.1128/msphere.00734-24
Cell aggregation mediated by ACE2 deletion in Candida auris modulates fungal colonization and host immune responses in the skin
Abstract
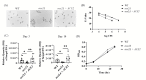
Candida auris is an emerging multi-drug-resistant fungal pathogen that colonizes the skin and causes invasive infections in hospitalized patients. Multi-cellular aggregative phenotype is widely reported in the C. auris isolates, but its role in skin colonization and host immune response is not yet known. In this study, we generated aggregative phenotype by deleting the ACE2 gene in C. auris and determined the fungal colonization and host immune response using an intradermal mouse model of C. auris skin infection. Our results indicate that mice infected with ace2Δ strain had significantly lower fungal load after 3 and 14 days post-infections compared to the non-aggregative wild-type and the ACE2 reintegrated strain. The colonization of ace2Δ is associated with increased recruitment of CD11b+ Ly6G+ neutrophils and decreased accumulation of CD11b+ Ly6 Chi inflammatory monocytes and CD11b+ MHCII+ CD64+ macrophages. Furthermore, Th17 cells and type 3 innate lymphoid cells (ILCs) were significantly increased in the skin tissue of ace2Δ infected mice. Our findings suggest that aggregative phenotype mediated by ACE2 deletion in C. auris induces potent neutrophil and IL-17-mediated immune response and reduces fungal colonization in the skin.IMPORTANCEC. auris is a rapidly emerging fungal pathogen that can colonize hospitalized patients, especially in skin tissue, and cause invasive infections. C. auris isolates exhibit morphological heterogeneity, and the multicellular aggregative phenotype of C. auris is reported frequently in clinical settings. Understanding the role of fungal morphotypes in colonization, persistence, and immune response in the skin microenvironment will have potential applications in clinical diagnosis and novel preventive and therapeutic measures. Here, we utilized the murine model of intradermal infection and determined that the aggregative phenotype of C. auris as the result of ACE2 gene deletion elicits potential innate and adaptive immune responses in mice. These observations will help explain the differences in the skin colonization and immune responses of the aggregative morphotype of C. auris and open the door to developing novel antifungal therapeutics.
Keywords: Candida auris; cell aggregation; colonization; skin immune response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Yeast and filamentous Candida auris stimulate distinct immune responses in the skin.mSphere. 2024 Jul 30;9(7):e0005524. doi: 10.1128/msphere.00055-24. Epub 2024 Jun 21. mSphere. 2024. PMID: 38904381 Free PMC article.
-
Intradermal infection and dissemination of Candida auris in immunocompetent and immunocompromised mouse models.Microbiol Spectr. 2024 Aug 6;12(8):e0012724. doi: 10.1128/spectrum.00127-24. Epub 2024 Jun 24. Microbiol Spectr. 2024. PMID: 38912805 Free PMC article.
-
Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies.Cell Host Microbe. 2021 Feb 10;29(2):210-221.e6. doi: 10.1016/j.chom.2020.12.002. Epub 2020 Dec 31. Cell Host Microbe. 2021. PMID: 33385336 Free PMC article.
-
Innate immune response to Candida auris.Curr Opin Microbiol. 2024 Aug;80:102510. doi: 10.1016/j.mib.2024.102510. Epub 2024 Jul 3. Curr Opin Microbiol. 2024. PMID: 38964276 Review.
-
Role of Microbiota in the Skin Colonization of Candida auris.mSphere. 2023 Feb 21;8(1):e0062322. doi: 10.1128/msphere.00623-22. Epub 2023 Jan 25. mSphere. 2023. PMID: 36695588 Free PMC article. Review.
References
-
- CDC . 2019. Antibiotic resistance threats in the United States, 2019. 2019. US Department of Health and Human Services, Centres for Disease Control and Prevention, Atlanta, GA.
-
- Sati H. 2022. WHO fungal priority pathogens list to guide research, development and public health action. World Health Organization: Geneva, Switzerland.
-
- Huang X, Hurabielle C, Drummond RA, Bouladoux N, Desai JV, Sim CK, Belkaid Y, Lionakis MS, Segre JA. 2021. Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies. Cell Host Microbe 29:210–221. doi:10.1016/j.chom.2020.12.002 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

