Pulmonary embolism after SARS-CoV-2 vaccination
- PMID: 39474208
- PMCID: PMC11513630
- DOI: 10.1016/j.jvacx.2024.100571
Pulmonary embolism after SARS-CoV-2 vaccination
Abstract
Background: During the COVID-19 vaccination campaign in Sweden, pulmonary embolism (PE) was a frequently reported suspected serious adverse drug reaction. The aim was to estimate risk of PE following vaccination for COVID-19 in the Swedish population aged 18 to 84 years.
Methods: Population-based cohort study using the CoVacSafe-SE established platform including national registers. PE-case definition: Individuals discharged from inpatient-care or visiting specialized outpatient-care with a main diagnosis of PE occurring between 27-Dec-2020 and 31-Dec-2022 without simultaneous diagnosis of COVID-19 infection. Time-to-event analysis was performed using multi-variable Cox' proportional hazard's models. Hazard Ratios (HR) adjusted for age, sex and co-morbidities were modelled.The vaccines were BNT162b2/Comirnaty®, mRNA1273/Spikevax® and ChAdOx1 nCoV-19/Vaxzevria® without regard to variants. Doses number one to five were studied.
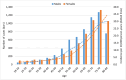
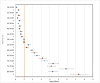
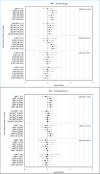
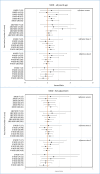
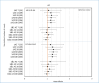
Results: Eighty percent of the study-population (≈6.1 million people) received at least two doses of COVID-19 vaccine. A total of 12,456 cases of PE were identified. Twenty-eight days after vaccinations we observed 99 cases after 701,455 1st doses of ChAdOx1 nCoV-19, HRadj, 1.29 (95%-CI, 1.05-1.59). Corresponding for BNT162b2 was 361 cases after 4,708,284 1st doses of BNT162b2 HRadj of 1.19 (95%-CI, 1.06-1.34) driven by age group 65-84; HR adj, 1.24 (95%-CI, 1.07-1.44). No increased risks were observed for mRNA1273.
Conclusion: In this nation-wide study, no strong associations were found between COVID-19 vaccinations and pulmonary embolism. Small increases in relative risk for the earliest doses of vaccines may be associated with prioritizing the frailest groups of people in the vaccination campaign, thus selection bias or unmeasured residual confounding is possible.
Keywords: Booster vaccinations; COVID-19 vaccines; Primary vaccinations; Public health; Pulmonary embolism; Regulatory science; Vaccine safety.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England.PLoS Med. 2022 Feb 22;19(2):e1003926. doi: 10.1371/journal.pmed.1003926. eCollection 2022 Feb. PLoS Med. 2022. PMID: 35192597 Free PMC article.
-
Evaluation of SARS-CoV-2 infection risks after primary vaccination with BNT162b2, BBIBP-CorV, or ChAdOx1-nCOV-19 and after homologous and heterologous booster vaccinations with these vaccines and evaluation of SARS-CoV-2 reinfection profiles.Biomedicine (Taipei). 2023 Sep 1;13(3):31-48. doi: 10.37796/2211-8039.1412. eCollection 2023. Biomedicine (Taipei). 2023. PMID: 37937059 Free PMC article.
-
Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: a double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial.Lancet Infect Dis. 2024 Apr;24(4):351-360. doi: 10.1016/S1473-3099(23)00650-3. Epub 2023 Dec 20. Lancet Infect Dis. 2024. PMID: 38141632 Clinical Trial.
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article. Review.
-
MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature.J Neurol. 2022 Oct;269(10):5198-5212. doi: 10.1007/s00415-022-11194-9. Epub 2022 Jun 23. J Neurol. 2022. PMID: 35737110 Free PMC article. Review.
References
-
- Katsoularis I., Fonseca-Rodríguez O., Farrington P., Jerndal H., Lundevaller E.H., Sund M., et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study. BMJ. 2022 Apr;6(377):e069590. PMID: 35387772. - PMC - PubMed
-
- Safiriyu I., Fatuyi M., Mehta A., Naser A., Alexander E., Vovan H., Shamaki G.R., Bob-Manuel T. Impact of COVID-19 Infection on the Clinical Outcomes of Pulmonary Embolism Hospitalizations : A Nationwide Analysis. Curr Probl Cardiol. 2023;48(7):101669. doi: 10.1016/j.cpcardiol.2023.101669. Epub 2023 Feb 23. PMID: 36841316. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

