Integrative analysis of gene expression, protein abundance, and metabolomic profiling elucidates complex relationships in chronic hyperglycemia-induced changes in human aortic smooth muscle cells
- PMID: 39473010
- PMCID: PMC11523773
- DOI: 10.1186/s13036-024-00457-w
Integrative analysis of gene expression, protein abundance, and metabolomic profiling elucidates complex relationships in chronic hyperglycemia-induced changes in human aortic smooth muscle cells
Abstract
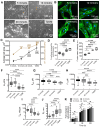
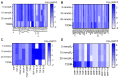
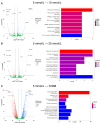
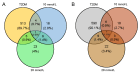
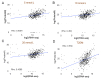
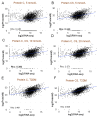
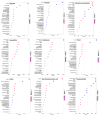
Type 2 diabetes mellitus (T2DM) is a major public health concern with significant cardiovascular complications (CVD). Despite extensive epidemiological data, the molecular mechanisms relating hyperglycemia to CVD remain incompletely understood. We here investigated the impact of chronic hyperglycemia on human aortic smooth muscle cells (HASMCs) cultured under varying glucose conditions in vitro, mimicking normal (5 mmol/L), pre-diabetic (10 mmol/L), and diabetic (20 mmol/L) conditions, respectively. Normal HASMC cultures served as baseline controls, and patient-derived T2DM-SMCs served as disease controls. Results showed significant increases in cellular proliferation, area, perimeter, and F-actin expression with increasing glucose concentration (p < 0.01), albeit not exceeding the levels in T2DM cells. Atomic force microscopy analysis revealed significant decreases in Young's moduli, membrane tether forces, membrane tension, and surface adhesion in SMCs at higher glucose levels (p < 0.001), with T2DM-SMCs being the lowest among all the cases (p < 0.001). T2DM-SMCs exhibited elevated levels of selected pro-inflammatory markers (e.g., ILs-6, 8, 23; MCP-1; M-CSF; MMPs-1, 2, 3) compared to glucose-treated SMCs (p < 0.01). Conversely, growth factors (e.g., VEGF-A, PDGF-AA, TGF-β1) were higher in SMCs exposed to high glucose levels but lower in T2DM-SMCs (p < 0.01). Pathway enrichment analysis showed significant increases in the expression of inflammatory cytokine-associated pathways, especially involving IL-10, IL-4 and IL-13 signaling in genes that are up-regulated by elevated glucose levels. Differentially regulated gene analysis showed that compared to SMCs receiving normal glucose, 513 genes were upregulated and 590 genes were downregulated in T2DM-SMCs; fewer genes were differentially expressed in SMCs receiving higher glucose levels. Finally, the altered levels in genes involved in ECM organization, elastic fiber synthesis and formation, laminin interactions, and ECM proteoglycans were identified. Growing literature suggests that phenotypic switching in SMCs lead to arterial wall remodeling (e.g., change in stiffness, calcific deposits formation), with direct implications in the onset of CVD complications. Our results suggest that chronic hyperglycemia is one such factor that leads to morphological, biomechanical, and functional alterations in vascular SMCs, potentially contributing to the pathogenesis of T2DM-associated arterial remodeling. The observed differences in gene expression patterns between in vitro hyperglycemic models and patient-derived T2DM-SMCs highlight the complexity of T2DM pathophysiology and underline the need for further studies.
Keywords: Biomechanics; Diabetes; Gene expression; Hyperglycemia; Pathway analysis; Transcriptomics; Vascular smooth muscle cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta.Dev Biol. 1996 Sep 15;178(2):430-45. doi: 10.1006/dbio.1996.0229. Dev Biol. 1996. PMID: 8830742
-
SPINT2 is involved in the proliferation, migration and phenotypic switching of aortic smooth muscle cells: Implications for the pathogenesis of thoracic aortic dissection.Exp Ther Med. 2023 Oct 9;26(6):546. doi: 10.3892/etm.2023.12245. eCollection 2023 Dec. Exp Ther Med. 2023. PMID: 37928510 Free PMC article.
-
Phenotype-based selection of bone marrow mesenchymal stem cell-derived smooth muscle cells for elastic matrix regenerative repair in abdominal aortic aneurysms.J Tissue Eng Regen Med. 2018 Jan;12(1):e60-e70. doi: 10.1002/term.2349. Epub 2017 Apr 25. J Tissue Eng Regen Med. 2018. PMID: 27860330
-
Bone morphogenetic protein-7 modulates genes that maintain the vascular smooth muscle cell phenotype in culture.J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S70-8. J Bone Joint Surg Am. 2001. PMID: 11263669 Review.
-
Potential role of tirzepatide towards Covid-19 infection in diabetic patients: a perspective approach.Inflammopharmacology. 2023 Aug;31(4):1683-1693. doi: 10.1007/s10787-023-01239-4. Epub 2023 May 19. Inflammopharmacology. 2023. PMID: 37208555 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

