Gut microbiome metabolites, molecular mimicry, and species-level variation drive long-term efficacy and adverse event outcomes in lung cancer survivors
- PMID: 39471749
- PMCID: PMC11550776
- DOI: 10.1016/j.ebiom.2024.105427
Gut microbiome metabolites, molecular mimicry, and species-level variation drive long-term efficacy and adverse event outcomes in lung cancer survivors
Abstract
Background: The influence of the gut microbiota on long-term immune checkpoint inhibitor (ICI) efficacy and immune-related adverse events (irAEs) is poorly understood, as are the underlying mechanisms.
Methods: We performed gut metagenome and metabolome sequencing of gut microbiotas from patients with lung cancer initially treated with anti-PD-1/PD-L1 therapy and explored the underlying mechanisms mediating long-term (median follow-up 1167 days) ICI responses and immune-related adverse events (irAEs). Results were validated in external, publicly-available datasets (Routy, Lee, and McCulloch cohorts).
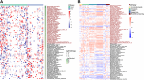
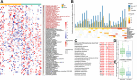
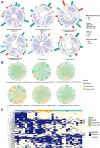
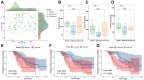
Findings: The ICI benefit group was enriched for propionate (P = 0.01) and butyrate/isobutyrate (P = 0.12) compared with the resistance group, which was validated in the McCulloch cohort (propionate P < 0.001, butyrate/isobutyrate P = 0.002). The acetyl-CoA pathway (P = 0.02) in beneficial species mainly mediated butyrate production. Microbiota sequences from irAE patients aligned with antigenic epitopes found in autoimmune diseases. Microbiotas of responsive patients contained more lung cancer-related antigens (P = 0.07), which was validated in the Routy cohort (P = 0.02). Escherichia coli and SGB15342 of Faecalibacterium prausnitzii showed strain-level variations corresponding to clinical phenotypes. Metabolome validation reviewed more abundant acetic acid (P = 0.03), propionic acid (P = 0.09), and butyric acid (P = 0.02) in the benefit group than the resistance group, and patients with higher acetic, propionic, and butyric acid levels had a longer progression-free survival and lower risk of tumor progression after adjusting for histopathological subtype and stage (P < 0.05).
Interpretation: Long-term ICI survivors have coevolved a compact microbial community with high butyrate production, and molecular mimicry of autoimmune and tumor antigens by microbiota contribute to outcomes. These results not only characterize the gut microbiotas of patients who benefit long term from ICIs but pave the way for "smart" fecal microbiota transplantation. Registered in the Chinese Clinical Trial Registry (ChiCTR2000032088).
Funding: This work was supported by Beijing Natural Science Foundation (7232110), National High Level Hospital Clinical Research Funding (2022-PUMCH-A-072, 2023-PUMCH-C-054), CAMS Innovation Fund for Medical Sciences (CIFMS) (2022-I2M-C&T-B-010).
Keywords: Gut microbiome; Immune checkpoint inhibitor; Lung cancer; Metabolites; Molecular mimicry.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Authors declare that they have no competing interests.
Figures


Similar articles
-
Dynamic gut microbiota changes in patients with advanced malignancies experiencing secondary resistance to immune checkpoint inhibitors and immune-related adverse events.Front Oncol. 2023 Apr 11;13:1144534. doi: 10.3389/fonc.2023.1144534. eCollection 2023. Front Oncol. 2023. PMID: 37114123 Free PMC article.
-
Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy.Int J Mol Sci. 2023 Feb 1;24(3):2769. doi: 10.3390/ijms24032769. Int J Mol Sci. 2023. PMID: 36769093 Free PMC article. Review.
-
The effects of antibiotics on the efficacy of immune checkpoint inhibitors in patients with non-small-cell lung cancer differ based on PD-L1 expression.Eur J Cancer. 2021 May;149:73-81. doi: 10.1016/j.ejca.2021.02.040. Epub 2021 Apr 7. Eur J Cancer. 2021. PMID: 33838391
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3 PMID: 33316104 Free PMC article. Updated.
-
Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer.Genome Med. 2021 Jun 23;13(1):107. doi: 10.1186/s13073-021-00923-w. Genome Med. 2021. PMID: 34162429 Free PMC article. Review.
References
-
- Hargadon K.M., Johnson C.E., Williams C.J. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. - PubMed
-
- Baumeister S.H., Freeman G.J., Dranoff G., Sharpe A.H. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–573. - PubMed
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

