Immune and non-immune mediators in the fibrosis pathogenesis of salivary gland in Sjögren's syndrome
- PMID: 39469708
- PMCID: PMC11513355
- DOI: 10.3389/fimmu.2024.1421436
Immune and non-immune mediators in the fibrosis pathogenesis of salivary gland in Sjögren's syndrome
Abstract
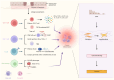
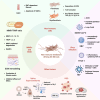
Sjögren's syndrome (SS) or Sjögren's disease (SjD) is a systemic autoimmune disease clinically manifested as sicca symptoms. This disease primarily impacts the functionality of exocrine glands, specifically the lacrimal and salivary glands (SG). SG fibrosis, an irreversible morphological change, is a severe consequence that occurs in the later stages of the disease due to sustained inflammation. However, the mechanism underlying SG fibrosis in SS remains under-investigated. Glandular fibrosis may arise from chronic sialadenitis, in which the interactions between infiltrating lymphocytes and epithelial cells potentially contributes to fibrotic pathogenesis. Thus, both immune and non-immune cells are closely involved in this process, while their interplays are not fully understood. The molecular mechanism of tissue fibrosis is partly associated with an imbalance of immune responses, in which the transforming growth factor-beta (TGF-β)-dependent epithelial-mesenchymal transition (EMT) and extracellular matrix remodeling are recently investigated. In addition, viral infection has been implicated in the pathogenesis of SS. Viral-specific innate immune response could exacerbate the autoimmune progression, resulting in overt inflammation in SG. Notably, post-COVID patients exhibit typical SS symptoms and severe inflammatory sialadenitis, which are positively correlated with SG damage. In this review, we discuss the immune and non-immune risk factors in SG fibrosis and summarize the evidence to understand the mechanisms upon autoimmune progression in SS.
Keywords: Sjögren’s disease (SjD); Sjögren’s syndrome; epithelial-mesenchymal transition; extracellular matrix remodeling; fibrosis; infection; salivary gland.
Copyright © 2024 Ma, Feng and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Interleukin-17 and -22 synergy linking inflammation and EMT-dependent fibrosis in Sjögren's syndrome.Clin Exp Immunol. 2019 Nov;198(2):261-272. doi: 10.1111/cei.13337. Epub 2019 Jun 5. Clin Exp Immunol. 2019. PMID: 31165469 Free PMC article.
-
Pathogenesis of Sjögren's syndrome-like autoimmune lesions in MRL/lpr mice.Pathol Int. 1994 Aug;44(8):559-68. doi: 10.1111/j.1440-1827.1994.tb01716.x. Pathol Int. 1994. PMID: 7952145 Review.
-
Chemokines Up-Regulated in Epithelial Cells Control Senescence-Associated T Cell Accumulation in Salivary Glands of Aged and Sjögren's Syndrome Model Mice.Int J Mol Sci. 2021 Feb 25;22(5):2302. doi: 10.3390/ijms22052302. Int J Mol Sci. 2021. PMID: 33669065 Free PMC article.
-
Exploring Salivary Epithelial Dysfunction in Sjögren's Disease.Int J Mol Sci. 2024 May 2;25(9):4973. doi: 10.3390/ijms25094973. Int J Mol Sci. 2024. PMID: 38732189 Free PMC article. Review.
-
Advances in cellular and molecular pathways of salivary gland damage in Sjögren's syndrome.Front Immunol. 2024 Jul 10;15:1405126. doi: 10.3389/fimmu.2024.1405126. eCollection 2024. Front Immunol. 2024. PMID: 39050857 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

