Development and epigenetic regulation of Atypical teratoid/rhabdoid tumors in the context of cell-of-origin and halted cell differentiation
- PMID: 39465218
- PMCID: PMC11502914
- DOI: 10.1093/noajnl/vdae162
Development and epigenetic regulation of Atypical teratoid/rhabdoid tumors in the context of cell-of-origin and halted cell differentiation
Abstract
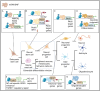
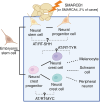
Atypical teratoid/rhabdoid tumors (AT/RTs) are aggressive brain tumors primarily observed in infants. The only characteristic, recurrent genetic aberration of AT/RTs is biallelic inactivation of SMARCB1 (or SMARCA4). These genes are members of the mSWI/SNF chromatin-remodeling complex, which regulates various developmental processes, including neural differentiation. This review explores AT/RT subgroups regarding their distinct SMARCB1 loss-of-function mechanisms, molecular features, and patient characteristics. Additionally, it addresses the ongoing debate about the oncogenic relevance of cell-of-origin, examining the influence of developmental stage and lineage commitment of the seeding cell on tumor malignancy and other characteristics. Epigenetic dysregulation, particularly through the regulation of histone modifications and DNA hypermethylation, has been shown to play an integral role in AT/RTs' malignancy and differentiation blockage, maintaining cells in a poorly differentiated state via the insufficient activation of differentiation-related genes. Here, the differentiation blockage and its contribution to malignancy are also explored in a cellular context. Understanding these mechanisms and AT/RT heterogeneity is crucial for therapeutic improvements against AT/RTs.
Keywords: INI1; cell differentiation; cell-of-origin; epigenetic regulation; pediatric tumor.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
None declared.
Figures


Similar articles
-
SWI/SNF complex heterogeneity is related to polyphenotypic differentiation, prognosis, and immune response in rhabdoid tumors.Neuro Oncol. 2020 Jun 9;22(6):785-796. doi: 10.1093/neuonc/noaa004. Neuro Oncol. 2020. PMID: 31912158 Free PMC article.
-
SMARCB1-deficient Tumors of Childhood: A Practical Guide.Pediatr Dev Pathol. 2018 Jan-Feb;21(1):6-28. doi: 10.1177/1093526617749671. Epub 2017 Dec 27. Pediatr Dev Pathol. 2018. PMID: 29280680 Review.
-
Two Cases of Atypical Teratoid/Rhabdoid Tumor in the Spinal Cord: Loss of SMARCB1 in a Child and Loss of SMARCA4 in an Adult.NMC Case Rep J. 2024 Jan 31;11:27-32. doi: 10.2176/jns-nmc.2022-0096. eCollection 2024. NMC Case Rep J. 2024. PMID: 38410173 Free PMC article.
-
Molecular targeted therapies for pediatric atypical teratoid/rhabdoid tumors.Pediatr Investig. 2022 May 23;6(2):111-122. doi: 10.1002/ped4.12325. eCollection 2022 Jun. Pediatr Investig. 2022. PMID: 35774526 Free PMC article. Review.
-
Functional relevance of genes predicted to be affected by epigenetic alterations in atypical teratoid/rhabdoid tumors.J Neurooncol. 2019 Jan;141(1):43-55. doi: 10.1007/s11060-018-03018-6. Epub 2018 Nov 16. J Neurooncol. 2019. PMID: 30446899
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics. CA Cancer J Clin. 2021;71(1):7–33. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
