Toxicological Comparison between Gold Nanoparticles in Different Shapes: Nanospheres Exhibit Less Hepatotoxicity and Lipid Dysfunction and Nanotriangles Show Lower Neurotoxicity
- PMID: 39464457
- PMCID: PMC11500156
- DOI: 10.1021/acsomega.4c05961
Toxicological Comparison between Gold Nanoparticles in Different Shapes: Nanospheres Exhibit Less Hepatotoxicity and Lipid Dysfunction and Nanotriangles Show Lower Neurotoxicity
Abstract
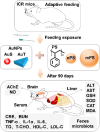
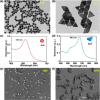
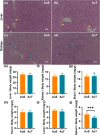
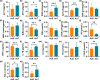
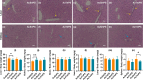
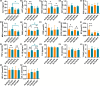
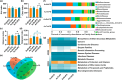
Gold nanoparticles (AuNPs) in different shapes have been developed and investigated for the treatment of various diseases. However, the potential toxicological vulnerability of different organs to morphologies of AuNPs and the complication of the toxicological profile of AuNPs by other health risk factors (e.g., plastic particles) have rarely been investigated systematically. Therefore, in this study, we aimed to investigate the toxicological differences between the spherical and triangular AuNPs (denoted as AuS and AuT, respectively) and the toxicological modulations by micro- or nanosized polystyrene plastic particles (denoted as mPS and nPS, respectively) in mice. Systemic biochemical characterizations were performed after a 90 day oral gavage feeding to obtain toxicological comparisons in different organs. In the case of single exposure to gold nanoparticles, AuT was associated with significantly higher aspartate amino-transferase (168.2%, P < 0.05), superoxide dismutase (183.6%, P < 0.001), catalase (136.9%, P < 0.01), total cholesterol (132.6%, P < 0.01), high-density lipoprotein cholesterol (131.3%, P < 0.05), and low-density lipoprotein cholesterol (204.6%, P < 0.01) levels than AuS. In contrast, AuS was associated with a significantly higher nitric oxide level (355.1%, P < 0.01) than AuT. Considering the overall toxicological profiles in single exposure and coexposure with multiscale plastics, it has been found that AuS is associated with lower hepatotoxicity and lipid metabolism malfunction, and AuT is associated with lower neurotoxicity than AuS. This finding may facilitate the future therapeutic design by considering the priority in protections of different organs and utilizing appropriate material morphologies.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Toxicity of gold nanoparticles complicated by the co-existence multiscale plastics.Front Microbiol. 2024 Aug 29;15:1447046. doi: 10.3389/fmicb.2024.1447046. eCollection 2024. Front Microbiol. 2024. PMID: 39268536 Free PMC article.
-
A Metabolomic Approach for the In Vivo Study of Gold Nanospheres and Nanostars after a Single-Dose Intravenous Administration to Wistar Rats.Nanomaterials (Basel). 2019 Nov 12;9(11):1606. doi: 10.3390/nano9111606. Nanomaterials (Basel). 2019. PMID: 31726761 Free PMC article.
-
Potassium bromate-induced nephrotoxicity and potential curative role of metformin loaded on gold nanoparticles.Sci Prog. 2021 Jul-Sep;104(3):368504211033703. doi: 10.1177/00368504211033703. Sci Prog. 2021. PMID: 34293965 Free PMC article.
-
Micro- and nanoplastic toxicity: A review on size, type, source, and test-organism implications.Sci Total Environ. 2023 Jun 20;878:162954. doi: 10.1016/j.scitotenv.2023.162954. Epub 2023 Mar 21. Sci Total Environ. 2023. PMID: 36948318 Review.
-
Gold nanoparticles functionalized with gadolinium-diethylenetriamine pentaacetic acid-cysteine conjugate.2010 Sep 24 [updated 2010 Nov 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Sep 24 [updated 2010 Nov 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21089255 Free Books & Documents. Review.
References
-
- Nambara K.; Niikura K.; Mitomo H.; Ninomiya T.; Takeuchi C.; Wei J.; Matsuo Y.; Ijiro K. Reverse Size Dependences of the cellular uptake of triangular and spherical gold nanoparticles. Langmuir 2016, 32 (47), 12559–12567. 10.1021/acs.langmuir.6b02064. - DOI - PubMed
- Han G.; Ghosh P.; Rotello V. M. Functionalized gold nanoparticles for drug delivery. Nanomedicine 2007, 2 (1), 113–123. 10.2217/17435889.2.1.113. - DOI - PubMed
- Paciotti G. F.; Myer L.; Weinreich D.; Goia D.; Pavel N.; McLaughlin R. E.; Tamarkin L. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Delivery 2004, 11 (3), 169–183. 10.1080/10717540490433895. - DOI - PubMed
-
- Jiang Y.; Horimoto N. N.; Imura K.; Okamoto H.; Matsui K.; Shigemoto R. Bioimaging with two-photon-induced luminescence from triangular nanoplates and nanoparticle aggregates of gold. Adv. Mater. 2009, 21, 2309–2313. 10.1002/adma.200802312. - DOI
-
- Yan L.; Mu J.; Ma P.; Li Q.; Yin P.; Liu X.; Cai Y.; Yu H.; Liu J.; Wang G.; et al. Gold nanoplates with superb photothermal efficiency and peroxidase-like activity for rapid and synergistic antibacterial therapy. Chem. Commun. 2021, 57 (9), 1133–1136. 10.1039/D0CC06925F. - DOI - PubMed
- Zharov V. P.; Mercer K. E.; Galitovskaya E. N.; Smeltzer M. S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys. J. 2006, 90 (2), 619–627. 10.1529/biophysj.105.061895. - DOI - PMC - PubMed
-
- Cardinal J.; Klune J. R.; Chory E.; Jeyabalan G.; Kanzius J. S.; Nalesnik M.; Geller D. A. Noninvasive radiofrequency ablation of cancer targeted by gold nanoparticles. Surgery 2008, 144 (2), 125–132. 10.1016/j.surg.2008.03.036. - DOI - PMC - PubMed
- Gannon C. J.; Patra C. R.; Bhattacharya R.; Mukherjee P.; Curley S. A. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J. Nanobiotechnol. 2008, 6, 2.10.1186/1477-3155-6-2. - DOI - PMC - PubMed
-
- Haes A. J.; Chang L.; Klein W. L.; Van Duyne R. P. Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. J. Am. Chem. Soc. 2005, 127 (7), 2264–2271. 10.1021/ja044087q. - DOI - PubMed
- Chamberland D. L.; Agarwal A.; Kotov N.; Brian Fowlkes J.; Carson P. L.; Wang X. Photoacoustic tomography of joints aided by an Etanercept-conjugated gold nanoparticle contrast agent-an ex vivo preliminary rat study. Nanotechnology 2008, 19 (9), 095101.10.1088/0957-4484/19/9/095101. - DOI - PubMed
- Bowman M. C.; Ballard T. E.; Ackerson C. J.; Feldheim D. L.; Margolis D. M.; Melander C. Inhibition of HIV fusion with multivalent gold nanoparticles. J. Am. Chem. Soc. 2008, 130 (22), 6896–6897. 10.1021/ja710321g. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
