Mechanisms of Germline Stem Cell Competition across Species
- PMID: 39459551
- PMCID: PMC11509876
- DOI: 10.3390/life14101251
Mechanisms of Germline Stem Cell Competition across Species
Abstract
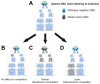
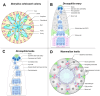
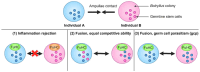
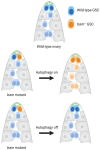
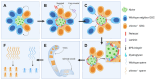
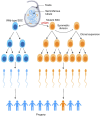
In this review, we introduce the concept of cell competition, which occurs between heterogeneous neighboring cell populations. Cells with higher relative fitness become "winners" that outcompete cells of lower relative fitness ("losers"). We discuss the idea of super-competitors, mutant cells that expand at the expense of wild-type cells. Work on adult stem cells (ASCs) has revealed principles of neutral competition, wherein ASCs can be stochastically lost and replaced, and of biased competition, in which a winning ASC with a competitive advantage replaces its neighbors. Germline stem cells (GSCs) are ASCs that are uniquely endowed with the ability to produce gametes and, therefore, impact the next generation. Mechanisms of GSC competition have been elucidated by studies in Drosophila gonads, tunicates, and the mammalian testis. Competition between ASCs is thought to underlie various forms of cancer, including spermatocytic tumors in the human testis. Paternal age effect (PAE) disorders are caused by de novo mutations in human GSCs that increase their competitive ability and make them more likely to be inherited, leading to skeletal and craniofacial abnormalities in offspring. Given its widespread effects on human health, it is important to study GSC competition to elucidate how cells can become winners or losers.
Keywords: cell competition; germline stem cell; mosaic analysis; ovary; paternal age affect disorders; stem cell competition; testis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Increased collective migration correlates with germline stem cell competition in a basal chordate.PLoS One. 2023 Oct 30;18(10):e0291104. doi: 10.1371/journal.pone.0291104. eCollection 2023. PLoS One. 2023. PMID: 37903140 Free PMC article.
-
Persistent competition among stem cells and their daughters in the Drosophila ovary germline niche.Development. 2009 Mar;136(6):995-1006. doi: 10.1242/dev.033340. Epub 2009 Feb 11. Development. 2009. PMID: 19211674
-
chinmo-mutant spermatogonial stem cells cause mitotic drive by evicting non-mutant neighbors from the niche.Dev Cell. 2022 Jan 10;57(1):80-94.e7. doi: 10.1016/j.devcel.2021.12.004. Epub 2021 Dec 22. Dev Cell. 2022. PMID: 34942115 Free PMC article.
-
Picking Winners and Losers: Cell Competition in Tissue Development and Homeostasis.Trends Genet. 2020 Jul;36(7):490-498. doi: 10.1016/j.tig.2020.04.003. Epub 2020 May 14. Trends Genet. 2020. PMID: 32418713 Free PMC article. Review.
-
Cell competition: the winners and losers of fitness selection.Development. 2019 Jul 5;146(13):dev167486. doi: 10.1242/dev.167486. Development. 2019. PMID: 31278123 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

