Hepatocellular Carcinoma in Mice Affects Neuronal Activity and Glia Cells in the Suprachiasmatic Nucleus
- PMID: 39457515
- PMCID: PMC11504045
- DOI: 10.3390/biomedicines12102202
Hepatocellular Carcinoma in Mice Affects Neuronal Activity and Glia Cells in the Suprachiasmatic Nucleus
Abstract
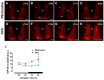
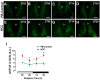
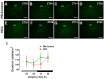
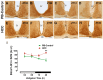
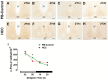
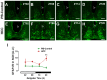
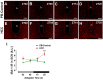
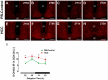
Background: Chronic liver diseases such as hepatic tumors can affect the brain through the liver-brain axis, leading to neurotransmitter dysregulation and behavioral changes. Cancer patients suffer from fatigue, which can be associated with sleep disturbances. Sleep is regulated via two interlocked mechanisms: homeostatic regulation and the circadian system. In mammals, the hypothalamic suprachiasmatic nucleus (SCN) is the key component of the circadian system. It generates circadian rhythms in physiology and behavior and controls their entrainment to the surrounding light/dark cycle. Neuron-glia interactions are crucial for the functional integrity of the SCN. Under pathological conditions, oxidative stress can compromise these interactions and thus circadian timekeeping and entrainment. To date, little is known about the impact of peripheral pathologies such as hepatocellular carcinoma (HCC) on SCN. Materials and Methods: In this study, HCC was induced in adult male mice. The key neuropeptides (vasoactive intestinal peptide: VIP, arginine vasopressin: AVP), an essential component of the molecular clockwork (Bmal1), markers for activity of neurons (c-Fos), astrocytes (GFAP), microglia (IBA1), as well as oxidative stress (8-OHdG) in the SCN were analyzed by immunohistochemistry at four different time points in HCC-bearing compared to control mice. Results: The immunoreactions for VIP, Bmal1, GFAP, IBA1, and 8-OHdG were increased in HCC mice compared to control mice, especially during the activity phase. In contrast, c-Fos was decreased in HCC mice, especially during the late inactive phase. Conclusions: Our data suggest that HCC affects the circadian system at the level of SCN. This involves an alteration of neuropeptides, neuronal activity, Bmal1, activation of glia cells, and oxidative stress in the SCN.
Keywords: AVP; HCC; SCN; VIP; circadian system; glia; oxidative stress.
Conflict of interest statement
The authors have no competing interests.
Figures
Similar articles
-
The transcription factor VAX1 in VIP neurons of the suprachiasmatic nucleus impacts circadian rhythm generation, depressive-like behavior, and the reproductive axis in a sex-specific manner in mice.Front Endocrinol (Lausanne). 2023 Dec 22;14:1269672. doi: 10.3389/fendo.2023.1269672. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38205198 Free PMC article.
-
Loss of arginine vasopressin- and vasoactive intestinal polypeptide-containing neurons and glial cells in the suprachiasmatic nucleus of individuals with type 2 diabetes.Diabetologia. 2019 Nov;62(11):2088-2093. doi: 10.1007/s00125-019-4953-7. Epub 2019 Jul 20. Diabetologia. 2019. PMID: 31327049 Free PMC article.
-
SCN VIP Neurons Are Essential for Normal Light-Mediated Resetting of the Circadian System.J Neurosci. 2018 Sep 12;38(37):7986-7995. doi: 10.1523/JNEUROSCI.1322-18.2018. Epub 2018 Aug 6. J Neurosci. 2018. PMID: 30082421 Free PMC article.
-
Roles of Neuropeptides, VIP and AVP, in the Mammalian Central Circadian Clock.Front Neurosci. 2021 Apr 15;15:650154. doi: 10.3389/fnins.2021.650154. eCollection 2021. Front Neurosci. 2021. PMID: 33935635 Free PMC article. Review.
-
[Mechanisms of structural plasticity associated with photic synchronization of the circadian clock within the suprachiasmatic nucleus].J Soc Biol. 2009;203(1):49-63. doi: 10.1051/jbio:2009004. Epub 2009 Apr 10. J Soc Biol. 2009. PMID: 19358811 Review. French.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

