Adipose-Derived Stem Cells as Carrier of Pro-Apoptotic Oncolytic Myxoma Virus: To Cross the Blood-Brain Barrier and Treat Murine Glioma
- PMID: 39457007
- PMCID: PMC11508294
- DOI: 10.3390/ijms252011225
Adipose-Derived Stem Cells as Carrier of Pro-Apoptotic Oncolytic Myxoma Virus: To Cross the Blood-Brain Barrier and Treat Murine Glioma
Abstract
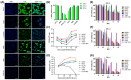
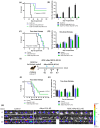
Treatment of glioblastoma is ineffective. Myx-M011L-KO/EGFP, a myxoma virus actively inducing apoptosis in BTICs linked to recurrence, offers innovative treatment. We loaded this construct into adipose-derived stem cells (ADSCs) to mitigate antiviral host responses and enable systemic delivery. The apoptotic and cytotoxic effects of the construct were studied using murine and human glioblastoma cell lines. Before implementing systemic delivery, we delivered the construct locally using ADSC to verify elimination of orthotopic murine glioma lesions. vMyx-M011L-KO/EGFP was cytotoxic to a murine cell line, preventing effective virus multiplication. In three human glioma cell lines, viral replication did occur, coupled with cell killing. The knock-out construct induced apoptotic cell death in these cultures. ADSCs infected ex vivo were shown to be sufficiently migratory to assure transfer of the therapeutic cargo to murine glioma lesions. Virus-loaded ADSCs applied to the artificial blood-brain barrier (BBB) yielded viral infection of glioma cells grown distally in the wells. Two rounds of local administration of this therapeutic platform starting 6 days post tumor implantation slowed down growth of orthotopic lesions and improved survival (total recovery < 20%). ADSCs infected ex vivo with vMyx-M011L-KO/EGFP show promise as a therapeutic tool in systemic elimination of glioma lesions.
Keywords: adipose tissue-derived stem cells (ADSCs); blood–brain barrier; glioblastoma; myxoma virus; oncolytic virotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Targeting IGF2 to reprogram the tumor microenvironment for enhanced viro-immunotherapy.Neuro Oncol. 2024 Sep 5;26(9):1602-1616. doi: 10.1093/neuonc/noae105. Neuro Oncol. 2024. PMID: 38853689 Free PMC article. Clinical Trial.
-
Combination Therapy with Secretome of Reovirus-Infected Mesenchymal Stem Cells and Metformin Improves Anticancer Effects of Irinotecan on Colorectal Cancer Cells in vitro.Intervirology. 2025;68(1):1-16. doi: 10.1159/000542356. Epub 2024 Nov 19. Intervirology. 2025. PMID: 39561737 Free PMC article.
-
Gambogic acid impairs the maintenance and therapeutic resistance of glioma stem cells by targeting B-cell-specific Moloney leukemia virus insert site 1.Phytomedicine. 2024 Dec;135:156070. doi: 10.1016/j.phymed.2024.156070. Epub 2024 Sep 17. Phytomedicine. 2024. PMID: 39326139
-
Antiangiogenic therapy for high-grade glioma.Cochrane Database Syst Rev. 2014 Sep 22;(9):CD008218. doi: 10.1002/14651858.CD008218.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Nov 22;11:CD008218. doi: 10.1002/14651858.CD008218.pub4. PMID: 25242542 Updated. Review.
-
Topical capsaicin (high concentration) for chronic neuropathic pain in adults.Cochrane Database Syst Rev. 2017 Jan 13;1(1):CD007393. doi: 10.1002/14651858.CD007393.pub4. Cochrane Database Syst Rev. 2017. PMID: 28085183 Free PMC article. Review.
References
-
- Pisklakova A., McKenzie B., Zemp F., Lun X., Kenchappa R.S., Etame A.B., Rahman M.M., Reilly K., Pilon-Thomas S., McFadden G., et al. M011L-deficient oncolytic myxoma virus induces apoptosis in brain tumor-initiating cells and enhances survival in a novel immunocompetent mouse model of glioblastoma. Neuro-Oncol. 2016;8:1088–1098. doi: 10.1093/neuonc/now006. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

