Alpha- to Beta-Cell Transdifferentiation in Neonatal Compared with Adult Mouse Pancreas in Response to a Modest Reduction in Beta-Cells Using Streptozotocin
- PMID: 39456933
- PMCID: PMC11508719
- DOI: 10.3390/ijms252011152
Alpha- to Beta-Cell Transdifferentiation in Neonatal Compared with Adult Mouse Pancreas in Response to a Modest Reduction in Beta-Cells Using Streptozotocin
Abstract
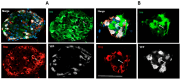
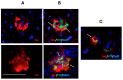
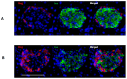
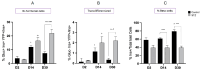
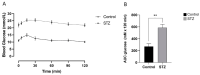
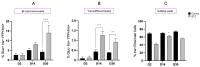
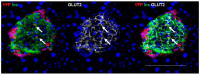
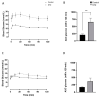
Following the near-total depletion of pancreatic beta-cells with streptozotocin (STZ), a partial recovery of beta-cell mass (BCM) can occur, in part due to the alpha- to beta-cell transdifferentiation with an intermediary insulin/glucagon bi-hormonal cell phenotype. However, human type 2 diabetes typically involves only a partial reduction in BCM and it is not known if recovery after therapeutic intervention involves islet cell transdifferentiation, or how this varies with age. Here, we used transgenic mouse models to examine if islet cell transdifferentiation contributes to BCM recovery following only a partial depletion of BCM. Cell lineage tracing was employed using Glucagon-Cre/yellow fluorescent protein (YFP) transgenic mice treated with STZ (25 mg/kg-neonates; 70 mg/kg-adults) or vehicle alone on 3 consecutive days. Mice were euthanized 2-30 days later with a prior glucose tolerance test on day 30, and immunofluorescence histology performed on the pancreata. Beta-cell abundance was reduced by 30-40% two days post STZ in both neonates and adults, and subsequently partially recovered in adult but not neonatal mice. Glucose tolerance recovered in adult females, but not in males or neonates. Bi-hormonal cell abundance increased 2-3-fold in STZ-treated mice vs. controls in both neonates and adults, as did transdifferentiated cells expressing insulin and the YFP lineage tag, but not glucagon. Transdifferentiated cell presence was an order of magnitude lower than that of bi-hormonal cells. We conclude that alpha- to beta-cell transdifferentiation occurs in mice following only a moderate depletion in BCM, and that this was accompanied by a partial recovery of BCM in adults.
Keywords: alpha-cell; beta-cell; bi-hormonal; diabetes; mouse; pancreas; streptozotocin; transdifferentiation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Increased alpha and beta cell mass during mouse pregnancy is not dependent on transdifferentiation.Exp Biol Med (Maywood). 2021 Mar;246(5):617-628. doi: 10.1177/1535370220972686. Epub 2020 Nov 24. Exp Biol Med (Maywood). 2021. PMID: 33231513 Free PMC article.
-
Dapagliflozin exerts positive effects on beta cells, decreases glucagon and does not alter beta- to alpha-cell transdifferentiation in mouse models of diabetes and insulin resistance.Biochem Pharmacol. 2020 Jul;177:114009. doi: 10.1016/j.bcp.2020.114009. Epub 2020 Apr 30. Biochem Pharmacol. 2020. PMID: 32360307
-
Effects of artemether on pancreatic islet morphology, islet cell turnover and α-cell transdifferentiation in insulin-deficient GluCreERT2;ROSA26-eYFP diabetic mice.J Pharm Pharmacol. 2022 Nov 25;74(12):1758-1764. doi: 10.1093/jpp/rgac075. J Pharm Pharmacol. 2022. PMID: 36206181
-
Pancreatic acinar-to-beta cell transdifferentiation in vitro.Front Biosci. 2008 May 1;13:5824-37. doi: 10.2741/3119. Front Biosci. 2008. PMID: 18508625 Review.
-
Role of transcription factors in the transdifferentiation of pancreatic islet cells.J Mol Endocrinol. 2015 Apr;54(2):R103-17. doi: 10.1530/JME-14-0290. J Mol Endocrinol. 2015. PMID: 25791577 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

