Mitochondrial Dysfunction as a Potential Mechanism Mediating Cardiac Comorbidities in Parkinson's Disease
- PMID: 39456761
- PMCID: PMC11507255
- DOI: 10.3390/ijms252010973
Mitochondrial Dysfunction as a Potential Mechanism Mediating Cardiac Comorbidities in Parkinson's Disease
Abstract
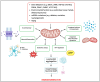
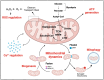
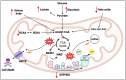
Individuals diagnosed with Parkinson's disease (PD) often exhibit heightened susceptibility to cardiac dysfunction, reflecting a complex interaction between these conditions. The involvement of mitochondrial dysfunction in the development and progression of cardiac dysfunction and PD suggests a plausible commonality in some aspects of their molecular pathogenesis, potentially contributing to the prevalence of cardiac issues in PD. Mitochondria, crucial organelles responsible for energy production and cellular regulation, play important roles in tissues with high energetic demands, such as neurons and cardiac cells. Mitochondrial dysfunction can occur in different and non-mutually exclusive ways; however, some mechanisms include alterations in mitochondrial dynamics, compromised bioenergetics, biogenesis deficits, oxidative stress, impaired mitophagy, and disrupted calcium balance. It is plausible that these factors contribute to the increased prevalence of cardiac dysfunction in PD, suggesting mitochondrial health as a potential target for therapeutic intervention. This review provides an overview of the physiological mechanisms underlying mitochondrial quality control systems. It summarises the diverse roles of mitochondria in brain and heart function, highlighting shared pathways potentially exhibiting dysfunction and driving cardiac comorbidities in PD. By highlighting strategies to mitigate dysfunction associated with mitochondrial impairment in cardiac and neural tissues, our review aims to provide new perspectives on therapeutic approaches.
Keywords: Parkinson’s disease (PD); cardiac dysfunction; mitochondria; mitochondrial dysfunction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
The interplay of neuronal mitochondrial dynamics and bioenergetics: implications for Parkinson's disease.Neurobiol Dis. 2013 Mar;51:43-55. doi: 10.1016/j.nbd.2012.05.015. Epub 2012 Jun 2. Neurobiol Dis. 2013. PMID: 22668779 Free PMC article. Review.
-
Current perspective of mitochondrial biology in Parkinson's disease.Neurochem Int. 2018 Jul;117:91-113. doi: 10.1016/j.neuint.2018.03.001. Epub 2018 Mar 14. Neurochem Int. 2018. PMID: 29550604 Free PMC article. Review.
-
Mitochondrial and Organellar Crosstalk in Parkinson's Disease.ASN Neuro. 2021 Jan-Dec;13:17590914211028364. doi: 10.1177/17590914211028364. ASN Neuro. 2021. PMID: 34304614 Free PMC article.
-
Mitochondrial Dysfunction and Parkinson's Disease: Pathogenesis and Therapeutic Strategies.Neurochem Res. 2023 Aug;48(8):2285-2308. doi: 10.1007/s11064-023-03904-0. Epub 2023 Mar 21. Neurochem Res. 2023. PMID: 36943668 Review.
References
-
- Park J.-H., Kim D.-H., Park Y.-G., Kwon D.-Y., Choi M., Jung J.-H., Han K. Association of Parkinson Disease with Risk of Cardiovascular Disease and All-Cause Mortality: A Nationwide, Population-Based Cohort Study. Circulation. 2020;141:1205–1207. doi: 10.1161/CIRCULATIONAHA.119.044948. - DOI - PubMed
-
- Husnu D., Eftal Murat B., Hikmet H. Cardiac Effects of Parkinson’s Disease. Open J. Park. Dis. Treat. 2020;3:006–007. doi: 10.17352/ojpdt.000009. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

