Membrane Ruffles: Composition, Function, Formation and Visualization
- PMID: 39456754
- PMCID: PMC11507850
- DOI: 10.3390/ijms252010971
Membrane Ruffles: Composition, Function, Formation and Visualization
Abstract
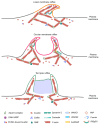
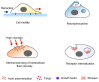
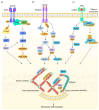
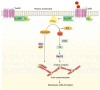
Membrane ruffles are cell actin-based membrane protrusions that have distinct structural characteristics. Linear ruffles with columnar spike-like and veil-like structures assemble at the leading edge of cell membranes. Circular dorsal ruffles (CDRs) have no supporting columnar structures but their veil-like structures, connecting from end to end, present an enclosed ring-shaped circular outline. Membrane ruffles are involved in multiple cell functions such as cell motility, macropinocytosis, receptor internalization, fluid viscosity sensing in a two-dimensional culture environment, and protecting cells from death in response to physiologically compressive loads. Herein, we review the state-of-the-art knowledge on membrane ruffle structure and function, the growth factor-induced membrane ruffling process, and the growth factor-independent ruffling mode triggered by calcium and other stimulating factors, together with the respective underlying mechanisms. We also summarize the inhibitors used in ruffle formation studies and their specificity. In the last part, an overview is given of the various techniques in which the membrane ruffles have been visualized up to now.
Keywords: calcium; cell motility; growth factor; macropinocytosis; membrane ruffles; optic imaging; viscosity sensing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Functions and regulation of circular dorsal ruffles.Mol Cell Biol. 2012 Nov;32(21):4246-57. doi: 10.1128/MCB.00551-12. Epub 2012 Aug 27. Mol Cell Biol. 2012. PMID: 22927640 Free PMC article. Review.
-
The mechanism of cell membrane ruffling relies on a phospholipase D2 (PLD2), Grb2 and Rac2 association.Cell Signal. 2011 Aug;23(8):1291-8. doi: 10.1016/j.cellsig.2011.03.010. Epub 2011 Mar 16. Cell Signal. 2011. PMID: 21419846 Free PMC article.
-
Every day I'm rufflin': Calcium sensing and actin dynamics in the growth factor-independent membrane ruffling of professional phagocytes.Small GTPases. 2017 Apr 3;8(2):65-70. doi: 10.1080/21541248.2016.1197873. Epub 2016 Jun 7. Small GTPases. 2017. PMID: 27267709 Free PMC article. Review.
-
Shear stress triggered circular dorsal ruffles formation to facilitate cancer cell migration.Arch Biochem Biophys. 2021 Sep 30;709:108967. doi: 10.1016/j.abb.2021.108967. Epub 2021 Jun 22. Arch Biochem Biophys. 2021. PMID: 34157295
-
Visualizing Membrane Ruffle Formation using Scanning Electron Microscopy.J Vis Exp. 2021 May 27;(171):10.3791/62658. doi: 10.3791/62658. J Vis Exp. 2021. PMID: 34125102 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

