In-silico evaluation of the T-cell based immune response against SARS-CoV-2 omicron variants
- PMID: 39455652
- PMCID: PMC11511884
- DOI: 10.1038/s41598-024-75658-w
In-silico evaluation of the T-cell based immune response against SARS-CoV-2 omicron variants
Abstract
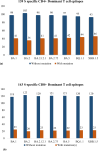
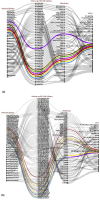
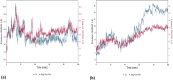
During of COVID-19 pandemic, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has continuously evolved, resulting in the emergence of several new variants of concerns (VOCs) with numerous mutations. These VOCs dominate in various regions due to increased transmissibility and antibody evasion, potentially reducing vaccine effectiveness. Nonetheless, it remains uncertain whether the recent SARS-CoV-2 VOCs have the ability to circumvent the T cell immunity elicited by either COVID-19 vaccination or natural infection. To address this, we conducted in-silico analysis to examine the impact of VOC-specific mutations at the epitope level and T cell cross-reactivity with the ancestral SARS-CoV-2. According to the in-silico investigation, T cell responses triggered by immunization or prior infections still recognize the variants in spite of mutations. These variants are expected to either maintain their dominant epitope HLA patterns or bind with new HLAs, unlike the epitopes of the ancestral strain. Our findings indicate that a significant proportion of immuno-dominant CD8 + and CD4 + epitopes are conserved across all the variants, implying that existing vaccines might maintain efficacy against new variations. However, further in-vitro and in-vivo studies are needed to validate the in-silico results and fully elucidate immune sensitivities to VOCs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Cross-protection induced by highly conserved human B, CD4+, and CD8+ T-cell epitopes-based vaccine against severe infection, disease, and death caused by multiple SARS-CoV-2 variants of concern.Front Immunol. 2024 Jan 22;15:1328905. doi: 10.3389/fimmu.2024.1328905. eCollection 2024. Front Immunol. 2024. PMID: 38318166 Free PMC article.
-
Preclinical evaluation of a synthetic peptide vaccine against SARS-CoV-2 inducing multiepitopic and cross-reactive humoral neutralizing and cellular CD4 and CD8 responses.Emerg Microbes Infect. 2021 Dec;10(1):1931-1946. doi: 10.1080/22221751.2021.1978823. Emerg Microbes Infect. 2021. PMID: 34538222 Free PMC article.
-
SARS-CoV-2 T Cell Responses Elicited by COVID-19 Vaccines or Infection Are Expected to Remain Robust against Omicron.Viruses. 2022 Jan 2;14(1):79. doi: 10.3390/v14010079. Viruses. 2022. PMID: 35062283 Free PMC article.
-
Degenerate CD8 Epitopes Mapping to Structurally Constrained Regions of the Spike Protein: A T Cell-Based Way-Out From the SARS-CoV-2 Variants Storm.Front Immunol. 2021 Sep 8;12:730051. doi: 10.3389/fimmu.2021.730051. eCollection 2021. Front Immunol. 2021. PMID: 34566990 Free PMC article. Review.
-
CD8+ T-cell immune escape by SARS-CoV-2 variants of concern.Front Immunol. 2022 Oct 27;13:962079. doi: 10.3389/fimmu.2022.962079. eCollection 2022. Front Immunol. 2022. PMID: 36389664 Free PMC article. Review.
References
-
- COVID - Coronavirus Statistics. Worldometer. https://www.worldometers.info/coronavirus/.
-
- Stern, A. & Andino, R. Viral Evolution. in Viral Pathogenesis 233–240 (Elsevier, 2016). 10.1016/B978-0-12-800964-2.00017-3.
-
- Markov, P. V. et al. The evolution of SARS-CoV-2. Nat. Rev. Microbiol.21, 361–379 (2023). - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

