ACE Inhibitors and Angiotensin Receptor Blockers for the Primary and Secondary Prevention of Cardiovascular Outcomes: Recommendations from the 2024 Egyptian Cardiology Expert Consensus in Collaboration with the CVREP Foundation
- PMID: 39455534
- PMCID: PMC11607301
- DOI: 10.1007/s40119-024-00381-6
ACE Inhibitors and Angiotensin Receptor Blockers for the Primary and Secondary Prevention of Cardiovascular Outcomes: Recommendations from the 2024 Egyptian Cardiology Expert Consensus in Collaboration with the CVREP Foundation
Abstract
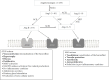
Introduction: The renin-angiotensin-aldosterone system (RAAS) plays a pivotal role in regulating blood pressure (BP), with dysregulation of RAAS resulting in hypertension and potentially heart failure (HF), myocardial infarction (MI), cardio-renal syndrome, and stroke. RAAS inhibitors, such as angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs), have advantages beyond BP control. However, differences between these two drug classes need to be considered when choosing a therapy for preventing cardiovascular events.
Methods: A panel of 36 Egyptian cardiologists developed consensus statements on RAAS inhibitors for primary and secondary prevention of cardiovascular outcomes and stroke, using a modified three-step Delphi process.
Results: The consensus statements highlight the importance of effective BP control and the role of RAAS blockade for prevention and management of various cardiovascular diseases. ACEis and ARBs differ in their mode of action and, thus, clinical effects. On the basis of available evidence, the consensus group recommended the following: ACEis should be considered as first choice (in preference to ARBs) to reduce the risk of MI, for primary prevention of HF, and for secondary prevention of stroke. ACEis and ARBs show equivalent efficacy for the primary prevention of stroke. Evidence also favors the preferential use of ACEis in patients with type 2 diabetes, for BP control, for the primary prevention of diabetic kidney disease, and to reduce the risk of major cardiovascular and renal outcomes. Treatment with an ACEi should be started within 24 h of ST segment elevation MI (and continued long term) in patients with HF, left ventricular systolic dysfunction, and/or diabetes. Angiotensin receptor/neprilysin inhibitors (ARNIs) are the first choice for patients with HF and reduced ejection fraction, with ACEis being the second choice in this group. ARBs are indicated as alternatives in patients who cannot tolerate ACEis. ACEis may be associated with cough development, but the incidence tends to be overestimated, and the risk can be reduced by use of a lipophilic ACEi or combining the ACEi with a calcium channel blocker.
Conclusion: RAAS blockade is an essential component of hypertension therapy; however, the protective effects provided by ACEis are superior to those of ARBs. Therefore, an ACEi is indicated in almost all cases, unless not tolerated.
Keywords: Angiotensin receptor blockers; Angiotensin-converting enzyme inhibitors; Cardiovascular outcomes; Heart failure; Hypertension; Myocardial infarction; Renin–angiotensin–aldosterone system; Stroke.
Plain language summary
Overstimulation of the renin–angiotensin–aldosterone system—a key regulator of blood pressure, and fluid and electrolyte balance—is known to cause an increase in blood pressure (also known as “hypertension”) and other diseases of the heart and blood vessels (the cardiovascular system). As such, treatments to block (or inhibit) this overstimulation are an essential part of medical strategies designed for the prevention of cardiovascular disease, especially in patients with hypertension (in whom the risk of death due to cardiovascular causes is high). Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are two types of medication that block overstimulation of the renin–angiotensin–aldosterone system, but they work in different ways. Angiotensin-converting enzyme inhibitors are superior to angiotensin receptor blockers after heart attacks (acute myocardial infarction), in patients with heart failure, for the prevention of stroke in individuals who have already had a stroke, and in patients with diabetes. Both types of medication have beneficial effects on the kidneys and associated outcomes, but only angiotensin-converting enzyme inhibitors have been shown to significantly reduce death due to cardiovascular causes, as well as death due to any cause. Overall, the protective effects of angiotensin-converting enzyme inhibitors on the heart are substantially greater than those of angiotensin receptor blockers, meaning that treatment with an angiotensin-converting enzyme inhibitor is preferred in all patients, except those who cannot tolerate the side effects of this drug class.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Mohamed Sobhy, Adel Eletriby, Hany Ragy, Hossam Kandil, Mohamed Ayman Saleh, Nabil Farag, Ramez Guindy, Ahmed Bendary, Ahmed Mohamed Elmahmoudy Nayel, Ahmed Shawky, Ayman Khairy, Ayman Mortada, Bassem Zarif, Haitham Badran, Hazem Khorshid, Kareem Mahmoud, Karim Said, Khaled Leon, Mahmoud Abdelsabour, Mazen Tawfik, Mohamed Aboel-Kassem F Abdelmegid, Mohamed Koriem, Mohamed Loutfi, Moheb Wadie, Mohamed Elnoamany, Mohamed Sadaka, Mohamed Seleem, Mohamed Zahran, Osama A. Amin, Sameh Elkaffas, Sherif Ayad, Wael El Kilany, Walid Ammar, Waleed Elawady, Walid Elhammady, and Yasser Abdelhady have nothing to disclose. Ethical Approval: As this study was classified as a consensus development technique and did not involve research on patients or patients’ data, obtaining approval from an ethics committee or internal review board was not necessary. All the clinicians who participated in this study are authors, who willingly served as panelists, agreed with the objectives of the modified Delphi panel study, and actively contributed to manuscript development. Doctors who filled in the Delphi questionnaire received an explanation about the project; they were informed about the intention to publish the results and were asked to complete the Delphi questionnaire if they agreed.
Figures

Similar articles
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Pharmacological treatments in panic disorder in adults: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 28;11(11):CD012729. doi: 10.1002/14651858.CD012729.pub3. Cochrane Database Syst Rev. 2023. PMID: 38014714 Free PMC article. Review.
References
-
- World Health Organization. Global report on hypertension: the race against a silent killer. 2023. WHO, Geneva, Switzerland. https://www.who.int/publications/i/item/9789240081062. Accessed Aug 13, 2024.
-
- Mancia G, Kreutz R, Brunström M, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41:1874–2071. - PubMed
-
- Unger T, Borghi C, Charchar F, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–57. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

