Assays for Assessing Mycobacterium avium Immunity and Evaluating the Effects of Therapeutics
- PMID: 39452774
- PMCID: PMC11510112
- DOI: 10.3390/pathogens13100903
Assays for Assessing Mycobacterium avium Immunity and Evaluating the Effects of Therapeutics
Abstract
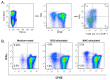
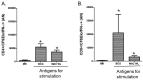
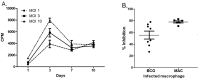
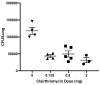
In Europe and North America, the prevalence of pulmonary nontuberculous mycobacteria (NTM) is increasing. Most pulmonary NTM infections are caused by the Mycobacterium avium complex (MAC). Sadly, the treatment of pulmonary MAC is suboptimal with failure rates ranging from 37% to 58%. Therefore, there is a need to develop new therapeutics. Developing new immunotherapies and studying their interaction with standard or new drugs requires reliable assays. Four different assays including CFSE-based flow cytometry, in vitro protection assays, IFN-γ ELISPOT, and murine infection models were optimized using a reference strain of MAC (ATCC 700898) to help with the development of immunotherapies for MAC. Expansion of proliferating and IFN-γ producing human T cells is optimal after 7 days of stimulation with MAC at a multiplicity of infection (MOI) of 0.1, achieving a stimulation index of 26.5 ± 11.6 (mean ± SE). The in vitro protection assay for MAC works best by co-culturing T cells expanded for 7 days with MAC (MOI 1)-infected autologous macrophages. Aerosol MAC infection of mice allows measurement of the effects of the BCG vaccine and clarithromycin. IFN-γ ELISPOT assays with live MAC (MOI 3) stimulation of splenocytes from mice immunized with BCG help identify differences between unimmunized mice and mice immunized with BCG. In conclusion, multiple assays are available for use to identify MAC-specific effector T cells, which will help in the development of new therapeutics or vaccines against pulmonary MAC.
Keywords: MAC; NTM; T cells; immunotherapy; macrophages; murine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
BCG Vaccination Induces M. avium and M. abscessus Cross-Protective Immunity.Front Immunol. 2019 Feb 19;10:234. doi: 10.3389/fimmu.2019.00234. eCollection 2019. Front Immunol. 2019. PMID: 30837992 Free PMC article.
-
[Strategies for Mycobacterium avium complex infection control in Japan: how do they improve the present situation?].Kekkaku. 2013 Mar;88(3):355-71. Kekkaku. 2013. PMID: 23672176 Japanese.
-
[Non-tuberculous mycobacteriosis. What has been coming out].Kekkaku. 2011 Feb;86(2):113-25. Kekkaku. 2011. PMID: 21404655 Japanese.
-
Diagnosis and Management of Pulmonary NTM with a Focus on Mycobacterium avium Complex and Mycobacterium abscessus: Challenges and Prospects.Microorganisms. 2022 Dec 23;11(1):47. doi: 10.3390/microorganisms11010047. Microorganisms. 2022. PMID: 36677340 Free PMC article. Review.
-
Mycobacterium avium complex and other nontuberculous mycobacteria in patients with HIV infection.Semin Respir Infect. 1989 Jun;4(2):123-32. Semin Respir Infect. 1989. PMID: 2664936 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

