AREG Upregulation in Cancer Cells via Direct Interaction with Cancer-Associated Fibroblasts Promotes Esophageal Squamous Cell Carcinoma Progression Through EGFR-Erk/p38 MAPK Signaling
- PMID: 39451251
- PMCID: PMC11506648
- DOI: 10.3390/cells13201733
AREG Upregulation in Cancer Cells via Direct Interaction with Cancer-Associated Fibroblasts Promotes Esophageal Squamous Cell Carcinoma Progression Through EGFR-Erk/p38 MAPK Signaling
Abstract
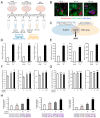
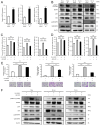
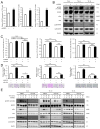
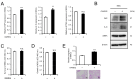
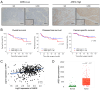
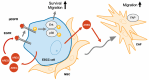
Cancer-associated fibroblasts (CAFs) are a key component of the tumor microenvironment and significantly contribute to the progression of various cancers, including esophageal squamous cell carcinoma (ESCC). Our previous study established a direct co-culture system of human bone marrow-derived mesenchymal stem cells (progenitors of CAFs) and ESCC cell lines, which facilitates the generation of CAF-like cells and enhances malignancy in ESCC cells. In this study, we further elucidated the mechanism by which CAFs promote ESCC progression using cDNA microarray analysis of monocultured ESCC cells and those co-cultured with CAFs. We observed an increase in the expression and secretion of amphiregulin (AREG) and the expression and phosphorylation of its receptor EGFR in co-cultured ESCC cells. Moreover, AREG treatment of ESCC cells enhanced their survival and migration via the EGFR-Erk/p38 MAPK signaling pathway. Immunohistochemical analysis of human ESCC tissues showed a positive correlation between the intensity of AREG expression at the tumor-invasive front and the expression level of the CAF marker FAP. Bioinformatics analysis confirmed significant upregulation of AREG in ESCC compared with normal tissues. These findings suggest that AREG plays a crucial role in CAF-mediated ESCC progression and could be a novel therapeutic target for ESCC.
Keywords: amphiregulin (AREG); cancer-associated fibroblasts (CAFs); direct co-culture; epidermal growth factor receptor (EGFR); esophageal squamous cell carcinoma (ESCC).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
FAP positive cancer-associated fibroblasts promote tumor progression and radioresistance in esophageal squamous cell carcinoma by transferring exosomal lncRNA AFAP1-AS1.Mol Carcinog. 2024 Oct;63(10):1922-1937. doi: 10.1002/mc.23782. Epub 2024 Jun 27. Mol Carcinog. 2024. PMID: 38934786
-
Fibroblast activation protein-positive fibroblasts promote tumor progression through secretion of CCL2 and interleukin-6 in esophageal squamous cell carcinoma.Lab Invest. 2019 Jun;99(6):777-792. doi: 10.1038/s41374-018-0185-6. Epub 2019 Jan 25. Lab Invest. 2019. PMID: 30683902
-
PAI-1 derived from cancer-associated fibroblasts in esophageal squamous cell carcinoma promotes the invasion of cancer cells and the migration of macrophages.Lab Invest. 2021 Mar;101(3):353-368. doi: 10.1038/s41374-020-00512-2. Epub 2020 Dec 12. Lab Invest. 2021. PMID: 33311557 Free PMC article.
-
Emerging role of cancer-associated fibroblasts in esophageal squamous cell carcinoma.Pathol Res Pract. 2024 Jan;253:155002. doi: 10.1016/j.prp.2023.155002. Epub 2023 Nov 30. Pathol Res Pract. 2024. PMID: 38056131 Review.
-
Cancer-associated fibroblasts: An emerging target against esophageal squamous cell carcinoma.Cancer Lett. 2022 Oct 10;546:215860. doi: 10.1016/j.canlet.2022.215860. Epub 2022 Aug 7. Cancer Lett. 2022. PMID: 35948121 Review.
References
-
- Morgan E., Soerjomataram I., Rumgay H., Coleman H.G., Thrift A.P., Vignat J., Laversanne M., Ferlay J., Arnold M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–658.e642. doi: 10.1053/j.gastro.2022.05.054. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

