Clinical validation of the suppressive impact of letrozole on liver fibrosis in patients with breast cancer undergoing continuous letrozole administration: A retrospective study
- PMID: 39446769
- PMCID: PMC11500940
- DOI: 10.1371/journal.pone.0311930
Clinical validation of the suppressive impact of letrozole on liver fibrosis in patients with breast cancer undergoing continuous letrozole administration: A retrospective study
Abstract
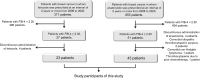
Treatment strategies for preventing liver fibrosis have not yet been established. Letrozole, widely used for breast cancer, has recently been reported to suppress liver fibrosis in murine models. Therefore, we aimed to validate the suppressive effects of letrozole on liver fibrosis in the clinical setting. From 2006 to 2020, 23 consecutive patients who received continuous letrozole treatment for 24 months or more and had a liver fibrosis marker FIB-4 index of ≥ 2.30, were included. Forty-three patients who underwent anastrozole treatment for 24 months or more and had a liver fibrosis marker FIB-4 index of ≥ 2.30, were also included as controls. The Fisher exact, chi-square, unpaired Student t, and paired Student t test were used to analyze the data. The patient characteristics were similar between the letrozole- and anastrozole-treated patient groups. Among the letrozole-treated patients, the mean FIB-4 index tended to decline during letrozole treatment; a significant decrease was observed at 18 and 24 months compared with the baseline values (p = 0.044 and p = 0.013). In addition, the mean aspartate aminotransferase-to-platelet ratio index (APRI) decreased during letrozole treatment; the values at 18 and 24 months were significantly lower than those at baseline (p = 0.024 and p = 0.026). In contrast, among anastrozole-treated patients, the mean FIB-4 index and APRI did not change during anastrozole treatment. When changes in the FIB-4 index were further examined in a limited number of patients with a FIB-4 index ≥ 2.67, a significant reduction in the FIB-4 index at 24 months compared with baseline was also observed in letrozole-treated patients (p = 0.023), but not in anastrozole-treated patients. In conclusion, our findings support a possible suppressive effect of letrozole on liver fibrosis in the clinical setting. Further studies are required to better understand the pharmacological effects of letrozole.
Copyright: © 2024 Ohkawa et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Authors Enomoto H and Tachiki H are employees of Towa Pharmaceutical Co., Ltd., the funder company of this study. They were merely involved in the review process in the writing of this study, as were the other authors. This does not alter the authors’ adherence to PLOS ONE policies on sharing data and materials.
Figures



Similar articles
-
Fibrosis index based on four factors better predicts advanced fibrosis or cirrhosis than aspartate aminotransferase/platelet ratio index in chronic hepatitis C patients.J Formos Med Assoc. 2015 Oct;114(10):923-8. doi: 10.1016/j.jfma.2015.07.004. Epub 2015 Aug 13. J Formos Med Assoc. 2015. PMID: 26279173
-
[A real-world study of the effects of endocrine therapy on liver function in breast cancer].Zhonghua Wai Ke Za Zhi. 2023 Feb 1;61(2):107-113. doi: 10.3760/cma.j.cn112139-20220925-00405. Zhonghua Wai Ke Za Zhi. 2023. PMID: 36720619 Chinese.
-
Changes in APRI and FIB-4 in HBeAg-negative treatment-naive chronic hepatitis B patients with significant liver histological lesions receiving 5-year entecavir therapy.Clin Exp Med. 2019 Aug;19(3):309-320. doi: 10.1007/s10238-019-00560-z. Epub 2019 May 20. Clin Exp Med. 2019. PMID: 31111345
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.Health Technol Assess. 2007 Jul;11(26):iii-iv, ix-xi, 1-134. doi: 10.3310/hta11260. Health Technol Assess. 2007. PMID: 17610808 Review.
-
Are all aromatase inhibitors the same? A review of controlled clinical trials in breast cancer.Clin Ther. 2005 Nov;27(11):1671-84. doi: 10.1016/j.clinthera.2005.11.013. Clin Ther. 2005. PMID: 16368441 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

