Metabolism: an important player in glioma survival and development
- PMID: 39436434
- PMCID: PMC11496451
- DOI: 10.1007/s12672-024-01402-5
Metabolism: an important player in glioma survival and development
Abstract
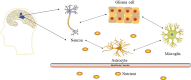
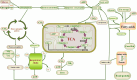
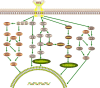
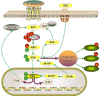
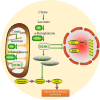
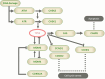
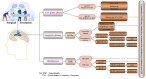
Gliomas are malignant tumors originating from both neuroglial cells and neural stem cells. The involvement of neural stem cells contributes to the tumor's heterogeneity, affecting its metabolic features, development, and response to therapy. This review provides a brief introduction to the importance of metabolism in gliomas before systematically categorizing them into specific groups based on their histological and molecular genetic markers. Metabolism plays a critical role in glioma biology, as tumor cells rely heavily on altered metabolic pathways to support their rapid growth, survival, and progression. Dysregulated metabolic processes, involving carbohydrates, lipids, and amino acids not only fuel tumor development but also contribute to therapy resistance and metastatic potential. By understanding these metabolic changes, key intervention points, such as mutations in genes like RTK, EGFR, RAS, and IDH can be identified, paving the way for novel therapeutic strategies. This review emphasizes the connection between metabolic pathways and clinical challenges, offering actionable insights for future research and therapeutic development in gliomas.
Keywords: Gliomas; Glucose metabolism; Lipid metabolism; Metabolisms; Signaling pathway; Treatment directions.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Tumor metabolism of malignant gliomas.Cancers (Basel). 2013 Nov 8;5(4):1469-84. doi: 10.3390/cancers5041469. Cancers (Basel). 2013. PMID: 24217114 Free PMC article.
-
Rediscovering Potential Molecular Targets for Glioma Therapy Through the Analysis of the Cell of Origin, Microenvironment and Metabolism.Curr Cancer Drug Targets. 2021;21(7):558-574. doi: 10.2174/1568009621666210504091722. Curr Cancer Drug Targets. 2021. PMID: 33949933 Review.
-
Alterations in the RTK/Ras/PI3K/AKT pathway serve as potential biomarkers for immunotherapy outcome of diffuse gliomas.Aging (Albany NY). 2021 Jun 8;13(11):15444-15458. doi: 10.18632/aging.203102. Epub 2021 Jun 8. Aging (Albany NY). 2021. PMID: 34100771 Free PMC article.
-
Altered metabolic landscape in IDH-mutant gliomas affects phospholipid, energy, and oxidative stress pathways.EMBO Mol Med. 2017 Dec;9(12):1681-1695. doi: 10.15252/emmm.201707729. EMBO Mol Med. 2017. PMID: 29054837 Free PMC article.
-
Gliomagenesis, Epileptogenesis, and Remodeling of Neural Circuits: Relevance for Novel Treatment Strategies in Low- and High-Grade Gliomas.Int J Mol Sci. 2024 Aug 16;25(16):8953. doi: 10.3390/ijms25168953. Int J Mol Sci. 2024. PMID: 39201639 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. - PubMed
-
- Wang LM, Englander ZK, Miller ML, Bruce JN. Malignant glioma. Adv Exp Med Biol. 2023;1405:1–30. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
